Transcutaneous auricular vagus nerve stimulation enhances structural and functional remodeling in sensorimotor networks following intracerebral hemorrhage in rats
- PMID: 40691625
- PMCID: PMC12278549
- DOI: 10.1186/s12984-025-01700-1
Transcutaneous auricular vagus nerve stimulation enhances structural and functional remodeling in sensorimotor networks following intracerebral hemorrhage in rats
Abstract
Background: Intracerebral hemorrhage (ICH) leads to severe neurological deficits by disrupting brain structure and function, particularly in the sensorimotor cortex. Effective interventions to promote post-ICH brain remodeling remain limited. This study investigated the effects of transcutaneous auricular vagus nerve stimulation (taVNS) on structural and functional remodeling in the sensorimotor networks of rats with ICH, using multi-scale analyses spanning micro-, meso-, and macro-levels.
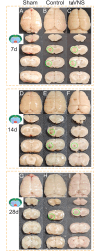
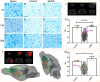
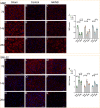
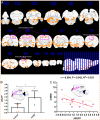
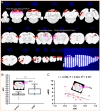
Methods: A rat model of left basal ganglia ICH was established, followed by taVNS intervention. Structural remodeling was assessed through histology, immunofluorescence, and transmission electron microscopy. Functional remodeling was evaluated using fractional amplitude of low-frequency fluctuations (fALFF) and degree centrality (DC) analyses.
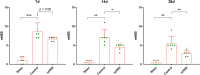
Results: taVNS enhanced myelin repair and axonal remodeling, indicated by increased myelin basic protein (MBP) expression, reduced dephosphorylated neurofilament protein (SMI-32), and partial restoration of synaptic ultrastructure. Functional imaging showed significant longitudinal increases in zfALFF and zDC values in sensorimotor regions, including the primary and secondary motor cortices, which negatively correlated with modified neurological severity scores (mNSS).
Conclusion: taVNS promotes structural and functional remodeling in the sensorimotor cortex after ICH, offering a potential therapeutic strategy for neurological recovery.
Keywords: Brain remodeling; Intracerebral hemorrhage; Neuroplasticity; Sensorimotor networks; TaVNS.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This study was approved by the Ethics Committee of the Laboratory Animal Science Department, Fudan University (202310008 S). Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Brainstem neuronal responses to transcutaneous auricular and cervical vagus nerve stimulation in rats.J Physiol. 2024 Aug;602(16):4027-4052. doi: 10.1113/JP286680. Epub 2024 Jul 19. J Physiol. 2024. PMID: 39031516 Free PMC article.
-
PENG-based self-powered transcutaneous auricular vagus nerve stimulation attenuated myocardial infarction-induced heart-brain remodeling via ameliorating the neuroinflammatory response in central amygdala.Int Immunopharmacol. 2025 Sep 23;162:115085. doi: 10.1016/j.intimp.2025.115085. Epub 2025 Jun 18. Int Immunopharmacol. 2025. PMID: 40836409
-
Baseline functional connectivity of the basal forebrain-cortical circuit predict taVNS treatment response in primary insomnia: a randomized controlled trial and fMRI study.BMC Med. 2025 Jul 9;23(1):412. doi: 10.1186/s12916-025-04126-7. BMC Med. 2025. PMID: 40629377 Free PMC article. Clinical Trial.
-
Transcutaneous auricular vagus nerve stimulation for epilepsy.Seizure. 2024 Jul;119:84-91. doi: 10.1016/j.seizure.2024.05.005. Epub 2024 May 30. Seizure. 2024. PMID: 38820674
-
Transcutaneous Auricular Vagus Nerve Stimulation for Managing Pain: A Scoping Review.Pain Manag Nurs. 2025 Feb;26(1):33-39. doi: 10.1016/j.pmn.2024.11.006. Epub 2024 Dec 16. Pain Manag Nurs. 2025. PMID: 39690039
References
MeSH terms
Grants and funding
- YSPTZX202033/Academician Innovation Platform Fund of Hainan Province
- SHDC22023224/Shanghai Shenkang Hospital Development Center-Municipal Hospital Diagnosis and Treatment Technology Promotion and Optimization Management Project
- 82471407/National Natural Science Foundation of China
- 82171382/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Miscellaneous

