"Small extracellular vesicles: messengers at the service of breast cancer agenda in the primary and distant microenvironments"
- PMID: 40691627
- PMCID: PMC12278669
- DOI: 10.1186/s13046-025-03471-y
"Small extracellular vesicles: messengers at the service of breast cancer agenda in the primary and distant microenvironments"
Abstract
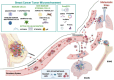
Breast cancer (BC) remains a leading cause of cancer-related mortality in women, with complex mechanisms driving its initiation, progression, and resistance to therapy. In recent years, the tumor microenvironment (TME) has gained attention for its critical role in shaping tumor behavior, where small extracellular vesicles (small EVs) have emerged as key mediators of intercellular communication. These vesicles carry a diverse cargo of proteins, lipids, DNA, and various non-coding RNAs-such as miR-21, miR-155, and miR-1246-mirroring the molecular status of their originating cells. This review highlights the roles of small EVs in immune modulation, stromal remodelling, and metastatic niche formation, emphasizing their contribution to therapy resistance and immune evasion. We discuss recent updates on EV biogenesis, characterisation and isolation techniques, such as ultracentrifugation, immunoaffinity and microfluidic systems. We also critically evaluate their potential for clinical application and how well they conform to the MISEV2023 guidelines. Furthermore, we examine small EVs as diagnostic tools in liquid biopsies and compare them with conventional methods such as mammography and tissue biopsies. We also discuss organotropism mediated by small EV cargo (e.g., integrins α6β4, αvβ5) and the diagnostic potential of protein and lipid signatures (e.g., PD-L1, CD63, and exosomal lipidomics). Therapeutically, we explore engineered small EVs for drug delivery, gene modulation, and immune activation, addressing challenges of targeting efficiency, in vivo stability, immunogenicity, and clinical scalability. The review discusses ongoing clinical trials involving small EVs in BC and highlights key translational gaps between preclinical advances and clinical implementation. Finally, we explores how integrating artificial intelligence, single-cell transcriptomics, and multi-omics approaches can help overcome major challenges such as small EV heterogeneity and tracking limitations. Crucially, this integration enables a more tailored understanding of each patient's tumor biology, reducing therapeutic failures by guiding more personalized and effective treatment strategies. Overall, small EVs represent a transformative tool in precision oncology, contingent on resolving key challenges in their clinical translation.
Keywords: Breast cancer; Immune cells; MicroRNAs; Microenvironment; Small extracellular vesicles; Stroma cells.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Unveiling the Involvement of Extracellular Vesicles in Breast Cancer's Organotrophic Metastasis: Molecular Mechanisms and Translational Prospects.Int J Mol Sci. 2025 Jun 6;26(12):5430. doi: 10.3390/ijms26125430. Int J Mol Sci. 2025. PMID: 40564894 Free PMC article. Review.
-
Diverse Populations of Extracellular Vesicles with Opposite Functions during Herpes Simplex Virus 1 Infection.J Virol. 2021 Feb 24;95(6):e02357-20. doi: 10.1128/JVI.02357-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33361424 Free PMC article.
-
Intercellular communication between extracellular vesicles from conditioned macrophages and breast cancer cells drives endocrine therapy resistance.Front Cell Dev Biol. 2025 Jun 3;13:1548724. doi: 10.3389/fcell.2025.1548724. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40567500 Free PMC article.
-
Isolation and characterization of bone mesenchymal cell small extracellular vesicles using a novel mouse model.J Bone Miner Res. 2024 Oct 29;39(11):1633-1643. doi: 10.1093/jbmr/zjae135. J Bone Miner Res. 2024. PMID: 39173022
-
Extracellular vesicles: the "Trojan Horse" within breast cancer host microenvironments.Mol Cancer. 2025 Jun 23;24(1):183. doi: 10.1186/s12943-025-02358-y. Mol Cancer. 2025. PMID: 40551109 Free PMC article. Review.
References
-
- Orrantia-Borunda E, Anchondo-Nuñez P, Acuña-Aguilar LE, Gómez-Valles FO, Ramírez-Valdespino CA. Subtypes of Breast Cancer. In: Mayrovitz HN, editor. Breast Cancer [Internet]. Brisbane (AU): Exon Publications; 2022 [cited 2025 Mar 28]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK583808/ - PubMed
-
- Dong Y, Chen J, Chen Y, Liu S. Targeting the STAT3 oncogenic pathway: Cancer immunotherapy and drug repurposing. Biomed Pharmacother Biomedecine Pharmacother. 2023;167:115513. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

