Lived experiences of adolescents with anorexia nervosa and their parents during olanzapine treatment: a qualitative study
- PMID: 40691644
- PMCID: PMC12281770
- DOI: 10.1186/s40337-025-01327-6
Lived experiences of adolescents with anorexia nervosa and their parents during olanzapine treatment: a qualitative study
Abstract
Background: Olanzapine is commonly prescribed in Anorexia Nervosa, despite conflicting evidence regarding its efficacy and safety. This study aimed to complement existent quantitative by exploring the lived experiences of adolescents and parents with this treatment.
Method: Ten adolescent girls and ten parents were interviewed in a single psychiatric department in Paris. Data saturation was achieved. We used semi-structured interviews enriched with narrative methods, including writing tasks and picture elicitation questions.
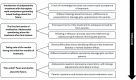
Results: Four chronological meta-themes were identified: (1) Introduction of antipsychotic treatment with Olanzapine: rapid acceptance generating mixed feelings about the future. (2) The first few weeks of medication: Adolescents questioning alone the treatment after first intakes. (3) Taking note of the results during the initial few months of treatment. (4) Then what? Fears and doubts about the future.
Conclusion: The prescription of olanzapine was often perceived as a liminal stage-a transitional moment akin to a "rite of passage" in the trajectory of Anorexia Nervosa. For many adolescents, it represented an opportunity to assert autonomy and gain a sense of empowerment in their relationships with both the medical team and their parents. However, implicit concerns about weight gain as a side effect often became a core issue of miscommunication. These concerns should be openly addressed throughout the treatment process. Shortening the usual duration of olanzapine therapy may offer a constructive path toward reinforcing the therapeutic alliance and avoiding non-medical discontinuation.
Keywords: Adolescents; Anorexia nervosa; Atypical antipsychotics; Child and adolescent psychiatry; Eating disorders; Neuroleptics; Olanzapine; Qualitative study.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures involving patients and professionals were performed is in accordance with the declaration of Helsinki regarding the principles for medical research involving human subjects. The protocol has received a favorable ethical opinion from the Comité de Protection des Personnes Nord-Ouest IV [IDRCB: 2022-A02141-42]. Consent for publication: All adolescents and their parents were informed about the study beforehand. The legal guardians of the adolescents and the participating parents provided their consent for both the study and the publication of the research findings. Competing interests: The authors declare no competing interests.
Figures
References
-
- Alhur A, Alhur AA, Alrkad E, Alshammari MH, Harbi N, Alshareef L, Alfaqeh M, Alayyafi T, Mohammed A, Alamri A, Alrowaebei H, Allehaibi L, Alosaimi A, Albishi R, AlThawwab M. Assessing the awareness of psychotropic medications among the Saudi population: benefits, risks, and prevalence. Cureus. 2024;16(8): e66818. 10.7759/cureus.66818. - DOI - PMC - PubMed
-
- Attia E, Kaplan AS, Walsh BT, Gershkovich M, Yilmaz Z, Musante D, et al. Olanzapine versus placebo for out-patients with anorexia nervosa. Psychol Med. 2011;41:2177–82. - PubMed
LinkOut - more resources
Full Text Sources