Molecular basis for the biosynthesis of the siderophore coprogen in the cheese-ripening fungus Penicillium roqueforti
- PMID: 40691645
- PMCID: PMC12278577
- DOI: 10.1186/s40659-025-00633-2
Molecular basis for the biosynthesis of the siderophore coprogen in the cheese-ripening fungus Penicillium roqueforti
Abstract
Background: Iron is an essential nutrient for microorganisms, including fungi, which have evolved strategies to acquire it. The most common strategy is the secretion of siderophores, low-molecular-weight compounds with a high affinity for ferric ions, which are involved in cellular iron uptake. Penicillium roqueforti, the fungus responsible for the ripening of blue-veined cheeses, produces coprogen, a hydroxamate-type siderophore. However, to date, the molecular basis for its biosynthesis remains elusive.
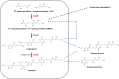
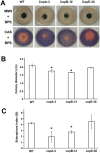
Results: In this study, we identified and characterized a biosynthetic gene cluster (BGC) responsible for coprogen biosynthesis in P. roqueforti, named the cop BGC. This BGC contains seven genes, three of which (copA, copB and copE) encode enzymes directly involved in coprogen biosynthesis from precursors molecules. Using CRISPR-Cas9, we targeted these three genes and analyzed the resulting mutants by Liquid Chromatography/High-Resolution Mass Spectrometry (LC/HRMS). Our results confirmed that all three genes are necessary for coprogen biosynthesis. Phenotypically, the mutants displayed growth differences under iron-deficient conditions, which correlated with their ability to either synthesize or fail to synthesize coprogen B and dimerumic acid, intermediates in the coprogen pathway with siderophore activity.
Conclusions: The results obtained in this work provide important insights into the molecular basis of coprogen biosynthesis in P. roqueforti, enhancing the understanding of how siderophores enable this fungus to thrive in iron-deficient environments.
Keywords: Penicillium roqueforti; Biosynthetic gene cluster; CRISPR-Cas9; Coprogen; Mass spectrometry; Siderophore.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures



References
-
- Frey PA, Reed GH. The ubiquity of iron. ACS Chem Biol. 2012;7:1477–81. - PubMed
-
- Heard AW, Bekker A, Kovalick A, Tsikos H, Ireland T, Dauphas N. Oxygen production and rapid iron oxidation in stromatolites immediately predating the Great Oxidation Event. Earth Planet Sci Lett. 2022;582: 117416.
-
- Khan A, Singh P, Srivastava A. Synthesis, nature and utility of universal iron chelator-siderophore: a review. Microbiol Res. 2018;212–213:103–11. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

