Clinical and radiographic evaluation of premixed bioceramic putty as an apical plug in nonvital immature anterior permanent teeth
- PMID: 40691703
- PMCID: PMC12280038
- DOI: 10.1038/s41598-025-11407-x
Clinical and radiographic evaluation of premixed bioceramic putty as an apical plug in nonvital immature anterior permanent teeth
Abstract
Achieving an apical seal is critical for apexification treatment of nonvital immature teeth. While this is commonly accomplished using biocompatible mineral trioxide aggregate (MTA), its limitations, such as prolonged setting time, discoloration, and challenging handling, have driven the search for alternative materials. This study aimed to compare the clinical and radiographic success of bioceramic putty Well-Root PT apical plug compared to MTA in the treatment of nonvital immature permanent incisors. Fifty immature nonvital maxillary permanent central incisors in thirty-eight children aged 8-11 years were randomly divided into two groups (25 teeth/group). Group I received MTA apical plugs, and Group II was treated with Well-Root PT apical plugs. Both groups were recalled at 6 and 12 months for clinical and radiographic evaluations. Statistical analysis was done for the gathered data. Both groups showed improved clinical signs and symptoms during all follow-up periods with no statistically significant difference. Regarding the periapical radiolucency (PAR) area, at twelve months, the mean PAR area in the Well-Root PT group was (0.14 ± 0.08) compared to (2.3 ± 0.9) in the MTA group, with highly statistically significant differences (p < 0.001). The mean periapical bone radiodensity in the Well-Root PT group was (178.2 ± 5.4) compared to (164.8 ± 9.4) in the MTA group at twelve-month follow-up, with highly statistically significant differences(p < 0.001). Well-Root PT, with its reduced technical sensitivity, demonstrates satisfactory clinical and radiographic success as an apical plug for nonvital immature permanent incisors compared to MTA.
Keywords: Apexification; Apical plugs; Mineral trioxide aggregate; Well-Root PT.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval and consent to participate: The ethical committee (REC), Faculty of Dentistry, Tanta University approved this study with code (#R-PED-11-23-3074) in accordance with the Helsinki Declaration of 1964 and its modifications. Written informed consent was obtained from all participants and their legal guardians. Consent for publication: Not applicable.
Figures
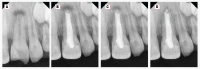
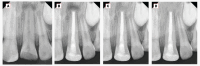
Similar articles
-
Treatment of open apex teeth using two types of white mineral trioxide aggregate after initial dressing with calcium hydroxide in children.Dent Traumatol. 2011 Jun;27(3):166-73. doi: 10.1111/j.1600-9657.2011.00984.x. Dent Traumatol. 2011. PMID: 21564517
-
Efficacy of mineral trioxide aggregate as an apical plug in non-vital young permanent teeth: preliminary results.J Clin Pediatr Dent. 2010 Winter;35(2):149-55. doi: 10.17796/jcpd.35.2.9061h7g718834017. J Clin Pediatr Dent. 2010. PMID: 21417116
-
A clinical evaluation of mineral trioxide aggregate for root-end closure of non-vital immature permanent incisors in children-a pilot study.Dent Traumatol. 2008 Feb;24(1):79-85. doi: 10.1111/j.1600-9657.2006.00485.x. Dent Traumatol. 2008. PMID: 18173672
-
Pulp treatment for extensive decay in primary teeth.Cochrane Database Syst Rev. 2018 May 31;5(5):CD003220. doi: 10.1002/14651858.CD003220.pub3. Cochrane Database Syst Rev. 2018. PMID: 29852056 Free PMC article.
-
Regenerative Endodontic Treatment or Mineral Trioxide Aggregate Apical Plug in Teeth with Necrotic Pulps and Open Apices: A Systematic Review and Meta-analysis.J Endod. 2017 Nov;43(11):1806-1820. doi: 10.1016/j.joen.2017.06.029. Epub 2017 Aug 16. J Endod. 2017. PMID: 28822564
References
-
- Yadav, A., Chak, R. K. & Khanna, R. Comparative evaluation of mineral trioxide aggregate, biodentine, and calcium phosphate cement in single visit apexification procedure for nonvital immature permanent teeth: a randomized controlled trial. Int. J. Clin. Pediatr. Dent.13 (Suppl 1), S1–S13. 10.5005/jp-journals-10005-1830 (2020). - PMC - PubMed
-
- Chugal, N., Mallya, S. M., Kahler, B. & Lin, L. M. Endodontic treatment outcomes. Dent. Clin. North. Am.61 (1), 59–80. 10.1016/j.cden.2016.08.009 (2017). - PubMed
-
- Zanjir, M. et al. Development of a Core Outcome Set for Endodontics (COS-ENDO). Part 5: COS-ENDO for studies of apexification and regenerative endodontics in permanent teeth. J Endod10.1016/j.joen.2025.01.012 (2025). - PubMed
-
- Santos, J. M. et al. Long-term outcome of nonvital immature permanent teeth treated with apexification and corono-radicular adhesive restoration: a case series. J. Endod. 48 (9), 1191–1199. 10.1016/j.joen.2022.06.007 (2022). - PubMed
-
- Kim, S. G., Malek, M., Sigurdsson, A., Lin, L. M. & Kahler, B. Regenerative endodontics: a comprehensive review. Int. Endod J.51 (12), 1367–1388. 10.1111/iej.12954 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

