Characterizing treatment interruptions in the OPERA cohort and virologic outcomes after resumption with bictegravir/emtricitabine/tenofovir alafenamide
- PMID: 40691801
- PMCID: PMC12281873
- DOI: 10.1186/s12981-025-00769-x
Characterizing treatment interruptions in the OPERA cohort and virologic outcomes after resumption with bictegravir/emtricitabine/tenofovir alafenamide
Abstract
Background: Despite advancements in antiretroviral therapy (ART) for people with HIV, barriers to adherence remain, potentially leading to long-term gaps in adherence known as treatment interruptions. These treatment interruptions are associated with viral rebound and can impact the effectiveness of the subsequent regimen and the long-term health of the individual. We aimed to characterize unplanned ART treatment interruptions in the OPERA® cohort and investigate virologic outcomes among individuals who resumed treatment with bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF).
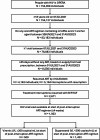
Methods: We identified adults with HIV-1 who were active in care and on an oral ART regimen with ≥ 2 antiretrovirals, including ≥ 1 anchor agent, between 30JUN2021 and 31AUG2023. Individuals with ≥ 1 period of ≥ 45 days without any ART, based on supply from last prescription, were considered to have experienced a treatment interruption. Individuals who resumed treatment by 31AUG2023 were defined as having experienced a treatment interruption with resumption. Each interruption observed during the study period was described, allowing for multiple interruptions per person. Treatment interruptions, pre-interruption regimens, and post-interruption regimens were described. Among individuals who resumed treatment with B/F/TAF, virologic outcomes were investigated through 29FEB2024 using Kaplan-Meier methods. All analyses were repeated with treatment interruption definitions of ≥ 60 and ≥ 90 days.
Results: Of 76,883 people for whom a treatment interruption could be observed, 8,550 (11%) experienced ≥ 1 period of ≥ 45 days without any ART. By 31AUG2023, 4,163 (49%) individuals resumed treatment (mean: 1.25 per person) and were included in the study population. The median age was 44 years, 81% were male, 52% Black, 41% White, and 18% Hispanic. Median time since HIV diagnosis was 118 months. B/F/TAF was the most common pre- and post-interruption regimen (49% and 51%, respectively). The cumulative probability of achieving virologic suppression on B/F/TAF was 68% (95% CI: 57, 78) when the viral load measurement was ≥ 200 copies/mL at resumption.
Conclusions: Treatment interruptions occurred in 11% of ART users in routine clinical care during the 26-month study period. Despite treatment interruption increasing the risk for viral rebound, most individuals who resumed treatment with B/F/TAF were able to achieve virologic suppression or avoid virologic failure.
Keywords: Antiretroviral therapy; B/F/TAF; Bictegravir/emtricitabine/tenofovir alafenamide; Cohort; HIV; Resumption; Treatment gap; Treatment interruption; Unplanned interruption; Virologic failure.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Institutional review board (IRB) approval covering patient data contained in the OPERA database was received from Advarra IRB; a waiver of informed consent and authorization for the use of protected health information for patient data was granted (Pro00023648). The study was conducted in accordance with HIPAA and HITECH requirements, which expand upon the ethical principles detailed in the 1964 Declaration of Helsinki. Consent for publication: Not applicable. Competing interests: KM, RKH, and GP are members of the Epidemiology and Clinical Advisory Board of Epividian. KM has received research grants from Gilead Sciences, Merck, Janssen, and GSK/ViiV Healthcare and honoraria for Speakers Bureau and Advisory Boards from Gilead Sciences, Merck, Janssen and GSK/ViiV Healthcare; and advisory board participation with Epividian. LB, MDO, JSF, and GPF are employed by Epividian, Inc.; Epividian has had research funded by AIDS Healthcare Foundation, EMD Serono, Gilead Sciences, Janssen Scientific Affairs, LLC, Merck & Co., Theratechnologies Inc., and ViiV Healthcare. RKH has received research grants from Gilead Sciences and ViiV Healthcare, speaker honoraria from ViiV Healthcare, Merck and Gilead Sciences, and advisory board participation with ViiV Healthcare, Gilead Sciences and Epividian. GP has received research funding paid to his institution from GSK/ViiV Healthcare, NIH/DAIDS, Gilead Sciences and Precision for Medicine, and is a member of the Epidemiology and Clinical Advisory Board for Epividian. NPM, JG, TL, and MD are employed by Gilead Sciences and hold stock in Gilead Sciences.
Figures
References
-
- Fauci AS, Lane HC. Four decades of HIV/AIDS — much accomplished, much to do. N Engl J Med. 2020;383(1):1–4. - PubMed
-
- Engler K, Lenart A, Lessard D, Toupin I, Lebouche B. Barriers to antiretroviral therapy adherence in developed countries: a qualitative synthesis to develop a conceptual framework for a new patient-reported outcome measure. AIDS Care. 2018;30(sup1):17–28. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous