Programmed cell death regulates hematopoietic cell homeostasis under radiation conditions
- PMID: 40691803
- PMCID: PMC12281764
- DOI: 10.1186/s13287-025-04502-3
Programmed cell death regulates hematopoietic cell homeostasis under radiation conditions
Abstract
Background: It is well-known that hematopoietic cells are sensitive to irradiation exposure. Apoptosis, necroptosis, pyroptosis and ferroptosis might contribute to irradiation-induced hematopoietic injury. However, it is uncertain whether different hematopoietic cells apply specific cell death pathways under irradiation exposure.
Methods: We investigated the role of different programmed cell death pathways in irradiation-induced hematopoietic cell injury. In order to study the acute and long-term effects of ionizing radiation on hematopoietic system, we established injury models of mice at different time points after irradiation and measured the proportion of hematopoietic stem progenitor cells by flow cytometry. The pattern of programmed cell death involved in radiation-induced hematopoietic cell injury was identified through the analysis of different populations of hematopoietic cells in the bone marrow by immunomagnetic bead sorting combined with qRT-PCR and flow cytometry. The role of pyroptosis in radiation injury of hematopoietic stem cells was further studied by Caspase-1 inhibitor VX-765 application. In vivo spleen colony formation, competitive bone marrow transplantation and secondary transplantation were used to verify the protective effect of inhibiting Caspase-1 on hematopoietic stem cells damaged by radiation. RNA sequencing (RNA-Seq) using Lin-c-Kit+ cell populations revealed the mechanism by which inhibition of Caspase-1 mitigates post-irradiation hematopoietic stem cell damage.
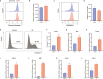
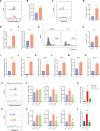
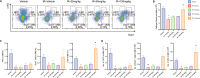
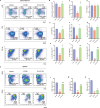
Results: A single exposure to whole-body ionizing radiation of 3 Gy causes acute bone marrow injury and long-term myelosuppression, resulting in hematopoietic imbalances and a bias toward myeloid differentiation. Ionizing radiation induced bone marrow B cell apoptosis and necroptosis, bone marrow T cell apoptosis. Various programmed cell death modes were involved in radiation injury of hematopoietic stem cells. Inhibition of Caspase-1 by VX-765 accelerated the recovery of hematopoietic stem cells after radiation. It is worth noting that inhibition of Caspase-1 promotes the proliferation and differentiation of hematopoietic stem cells after ionizing radiation. VX-765 treatment under ionizing radiation stress increased numbers of spleen colony formation, ability of long-term hematopoietic reconstitution in vivo and self-renewal. VX-765 alleviates post-irradiation hematopoietic stem cell injury by inhibiting pyroptosis, apoptosis and necroptosis.
Conclusions: These data suggest that multiple programmed cell death pathways are involved in radiation-induced damage to hematopoietic cells. Inhibiting Caspase-1 activity can be used as a strategy for protecting against radiation-induced injury to hematopoietic stem cells.
Keywords: Apoptosis; Hematopoietic cells; Ionizing radiation; Necroptosis; Pyroptosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study did not include clinical trials. All experiments were approved by the Experimental Animal Welfare and Ethics Committee of Nanchang University under the project “The role of activating exogenous apoptosis to remove aging hematopoietic stem cells in bone marrow reconstruction of radiation-induced chronic injury”. The approval number is NCU-CLA-2019-319. The approval date for these animal experiments is 2019-03-16. Our contributions are reported in accordance with the ARRIVE guidelines 2.0. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Advances in the Regulation of Hematopoietic Homeostasis by Programmed Cell Death Under Radiation Conditions.Stem Cell Rev Rep. 2025 May;21(4):935-952. doi: 10.1007/s12015-025-10863-2. Epub 2025 Mar 8. Stem Cell Rev Rep. 2025. PMID: 40056317
-
Leucine-rich pentatricopeptide repeat-containing protein (LRPPRC)-stabilized lncRNA small nucleolar RNA host gene 15 (Snhg15) modulates hematopoietic injury induced by γ-ray irradiation via m6A modification.Mol Biomed. 2025 Jun 25;6(1):44. doi: 10.1186/s43556-025-00279-2. Mol Biomed. 2025. PMID: 40555877 Free PMC article.
-
Thrombopoietin mimetic stimulates bone marrow vascular and stromal niches to mitigate acute radiation syndrome.Stem Cell Res Ther. 2024 Apr 29;15(1):123. doi: 10.1186/s13287-024-03734-z. Stem Cell Res Ther. 2024. PMID: 38679747 Free PMC article.
-
Metformin Modulates Oxidative Stress in Murine Mesenchymal Stem Cells In Vitro and Alleviates Corticosteroid-Induced Inflammation and Impairment of Bone Formation.HSS J. 2025 Jul 11:15563316251351031. doi: 10.1177/15563316251351031. Online ahead of print. HSS J. 2025. PMID: 40661872 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
-
- Gutschalk CM, Herold-Mende CC, Fusenig NE, Mueller MM. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor promote malignant growth of cells from head and neck squamous cell carcinomas in vivo. Cancer Res. 2006;66(16):8026–36. - PubMed
-
- Milyavsky M, Gan OI, Trottier M, Komosa M, Tabach O, Notta F, Lechman E, Hermans KG, Eppert K, Konovalova Z, et al. A distinctive DNA damage response in human hematopoietic stem cells reveals an apoptosis-independent role for p53 in self-renewal. Cell Stem Cell. 2010;7(2):186–97. - PubMed
-
- Wang J, Sun Q, Morita Y, Jiang H, Groß A, Lechel A, Hildner K, Guachalla LM, Gompf A, Hartmann D, et al. A differentiation checkpoint limits hematopoietic stem cell Self-Renewal in response to DNA damage. Cell. 2014;158(6):1444. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

