Combination of sibutramine and topiramate for the treatment of obesity: the SIBAMATE retrospective cohort study : Sibutramine and topiramate for the treatment of obesity
- PMID: 40691810
- PMCID: PMC12278575
- DOI: 10.1186/s13098-025-01842-1
Combination of sibutramine and topiramate for the treatment of obesity: the SIBAMATE retrospective cohort study : Sibutramine and topiramate for the treatment of obesity
Abstract
Background: Long-term treatment of obesity with lifestyle changes alone is unsustainable for most individuals. Antiobesity medications are recommended for individuals with a body mass index (BMI) ≥ 30 kg/m² or ≥ 27 kg/m² with one or more comorbidities. In Brazil, the prescription of combined sibutramine plus topiramate for obesity management is common in daily clinical practice. However, data on its effectiveness and safety are lacking. Thus, the objective of this study was to evaluate this combination for treating obesity in a real-world scenario.
Methods: This retrospective cohort study included individuals with obesity ≥ 18 years prescribed sibutramine and topiramate for at least 3 months between 2012 and 2022 at a large tertiary healthcare center. Baseline and follow-up data were collected from medical records.
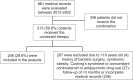
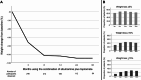
Results: Among 861 medical records screened, 246 (28.6%) were included. Most participants were female (86.2%) with a mean age of 42.8 ± 12.7 years, 52% had hypertension, 31.3% type 2 diabetes and 30% dyslipidemia. The average baseline BMI and weight were 39.7 kg/m² and 104.2 kg, respectively. The mean daily doses of sibutramine and topiramate were 11 ± 2.1 mg and 119.7 ± 54.7 mg, respectively. There was a significant change in body weight precociously at 3 months on the combination (96.8 ± 20.7 kg, p < 0.001), with 61.8% of patients achieving at least ≥ 5% of weight loss, 29.4% ≥10% and 10.9% ≥15%. At 36 months, 64% maintained at least ≥ 5% of weight loss, 40.6% ≥10% and 26.5% ≥15%. Common adverse effects included paresthesia, memory impairment, bradyphrenia and elevated blood pressure. The discontinuation rate was 24.4%. No major safety concern was observed in a mean follow-up of 25.3 months.
Conclusion: In a real-world setting, sibutramine and topiramate combination therapy was associated with clinically meaningful weight loss alongside a good tolerability profile.
Keywords: Antiobesity medication; Obesity; Sibutramine; Topiramate; Weight loss.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the local Ethics Committee. Since it is a retrospective trial based on medical records, informed consent was waived. Consent for publication: Not applicable since this is not a case report. Competing interests: The authors declare no competing interests. Conflict of interest: MAMS has received honoraria related to support for meetings or travel, speaker activities, or research from Novo Nordisk, Amgen, Eurofarma and Eli-Lilly. GNCF has received grants from Novo Nordisk and Eurofarma. EZK has received grants from Eurofarma. CC has served as advisory board for Novo Nordisk and reports having received research grants from Eli-Lilly, Novo Nordisk, Merck, Boehringer Ingelheim, Eurofarma, Fractyl, and EMS. MCM has received consulting fees, honoraria, and support for meetings or travel from Merck, Lilly, Novo Nordisk, Takeda, EMS, Eurofarma, and Aché. The other authors have nothing to disclose.
Figures
References
-
- World Obesity Atlas. 2024: Obesity and its consequences. World Obes Federation, 2024; 1:1–236 (accessed 8 December 2024). https://data.worldobesity.org/publications/WOF-Obesity-Atlas-v7.pdf
-
- Stumpf MAM, Cercato C, de Melo ME, et al. Down the rabbit hole: reviewing the evidence for primary prevention of cardiovascular disease in people with obesity. Eur J Prev Cardiol. 2023;30:1895–905. - PubMed
-
- Busetto L, Dicker D, Frühbeck G, et al. A new framework for the diagnosis, staging and management of obesity in adults. Nat Med. 2024;30:2395–9. - PubMed
-
- Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389:2221–32. - PubMed
LinkOut - more resources
Full Text Sources