Enhancing treatment outcomes after gynaecological cancer (ACUMEN): a randomised controlled exercise trial protocol
- PMID: 40691826
- PMCID: PMC12278665
- DOI: 10.1186/s12885-025-14298-3
Enhancing treatment outcomes after gynaecological cancer (ACUMEN): a randomised controlled exercise trial protocol
Abstract
Background: Treatment for gynaecological cancer often entails challenges that negatively affect quality-of-life. While exercise has been shown to improve physical and psychosocial well-being during cancer recovery, affected women often avoid exercise due to the specific health challenges they face after treatment. The Enhancing treatment outcomes after gynaecological cancer (ACUMEN) program aims to enhance health-related quality of life in this group by promoting lifelong exercise habits.
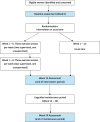
Methods: The ACUMEN trial is a single-blind, multi-centre randomised controlled trial that evaluates the impact of a structured exercise intervention on quality of life in women who have completed primary treatment for gynaecological cancer within the last 60 months. A total of 342 participants will be randomly assigned to either usual care or an intervention group. The intervention involves a 12-week personalised exercise program, where participants will complete three 1-h exercise sessions per week. During the first six weeks, participants will receive two supervised sessions with accredited exercise physiologists or physiotherapists and self-manage one session per week. In the following six weeks, this shifts to one supervised session and two self-managed sessions weekly, with goal of fostering sustainable, self-managed exercise habits. Assessments will take place at baseline, Week 12 (end of intervention), and Week 24 (end of maintenance). The primary outcome is health-related quality of life, measured by the Short Form-36. Secondary outcomes include exercise self-efficacy, body composition, symptoms of lymphoedema, physical fitness, biological markers, and habitual physical activity levels. For intervention group participants assigned accredited exercise physiologists or physiotherapists will track attendance, adherence, feasibility, and safety.
Discussion: The ACUMEN trial will evaluate whether a structured exercise program improves health-related quality of life in women recovering from gynaecological cancer treatment. The findings will help determine if the intervention is effective and sustainable, with a simultaneous cost-effectiveness analysis conducted to assess its value for money. Results from this study could inform future exercise-based interventions to enhance cancer recovery.
Trial registration: Australian New Zealand Clinical Trial Registry (ACTRN12621000050853, registered 19/01/2021).
Keywords: Cancer recovery; Exercise intervention; Gynaecological cancer; Quality of life; Self-efficacy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study has received ethical approval from the Royal Brisbane and Women’s Hospital Human Research Ethics Committee (reference number: REC/2020/QRBW/67832, protocol version and date at point of submission v1.11 dated 08/03/2024). Any protocol modifications will be approved by the Ethics Committee before implementation. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Australian Institute of Health and Welfare. Cancer data in Australia: AIHW, Australian Government; 2024. Available from: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents....
-
- Cancer Australia. National Framework for Gynaecological Cancer Control: Cancer Australia, Australian Government; 2016. Available from: https://www.canceraustralia.gov.au/publications-and-resources/cancer-aus....
-
- Pennington KP, McTiernan A. The role of physical activity in breast and gynecologic cancer survivorship. Gynecol Oncol. 2018;149(1):198–204. 10.1016/j.ygyno.2018.01.020. - PubMed
-
- Kokkinos P, Faselis C, Samuel IBH, Lavie CJ, Zhang J, Vargas JD, et al. Changes in cardiorespiratory fitness and survival in patients with or without cardiovascular disease. J Am Coll Cardiol. 2023;81(12):1137–47. 10.1016/j.jacc.2023.01.027. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

