Identifying Lanthionine Ketimine Derivatives for Maturation and Proliferative Effects in Oligodendrocyte Progenitor Cells
- PMID: 40692140
- PMCID: PMC12296138
- DOI: 10.1080/17590914.2025.2535963
Identifying Lanthionine Ketimine Derivatives for Maturation and Proliferative Effects in Oligodendrocyte Progenitor Cells
Abstract
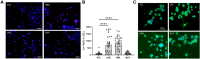
Previous studies have shown that lanthionine ketimine ethyl ester (LKE) reduces clinical scores in the experimental autoimmune encephalomyelitis (EAE) mouse model of Multiple Sclerosis, induces differentiation of oligodendrocyte progenitor cells (OPCs) in vitro, and accelerates remyelination following cuprizone induced demyelination. In a search for derivatives with greater efficacy to induce OPC maturation or proliferation, we screened a panel of 2-alkyl and 3-phosphonate substituted LK derivatives. Incubation of Oli-neu oligodendrocyte cells with 2-n-butyl- or 2-n-hexyl-LKE-phosphonate reduced spontaneous cell death, increased proliferation, and increased maturation. These were associated with changes in corresponding mRNA levels of Olig2, PLP, and O4. These derivatives also reduced cell death and increased proliferation and maturation in primary mouse OPCs. The increased hydrophobicity of these derivatives suggests these will be better candidates for testing effects in animal models of Multiple Sclerosis and other demyelinating diseases.
Keywords: Oli-neu cells; differentiation; myelin; oligodendrocyte; proliferation.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures








References
-
- Arimura, N., Ménager, C., Kawano, Y., Yoshimura, T., Kawabata, S., Hattori, A., Fukata, Y., Amano, M., Goshima, Y., Inagaki, M., Morone, N., Usukura, J., & Kaibuchi, K. (2005). Phosphorylation by Rho kinase regulates CRMP-2 activity in growth cones. Molecular and Cellular Biology, 25(22), 9973–9984. 10.1128/MCB.25.22.9973-9984.2005 - DOI - PMC - PubMed
-
- Boccazzi, M., Macchiarulo, G., Lebon, S., Janowska, J., Le Charpentier, T., Faivre, V., Hua, J., Marangon, D., Lecca, D., Fumagalli, M., Mani, S., Abbracchio, M. P., Gressens, P., Schang, A. L., & Van Steenwinckel, J. (2023). G protein-coupled receptor 17 is regulated by WNT pathway during oligodendrocyte precursor cell differentiation. Neurobiology of Disease, 187, 106315. 10.1016/j.nbd.2023.106315 - DOI - PubMed
-
- de Faria, O., Jr., Dhaunchak, A. S., Kamen, Y., Roth, A. D., Kuhlmann, T., Colman, D. R., & Kennedy, T. E. (2019). TMEM10 promotes oligodendrocyte differentiation and is expressed by oligodendrocytes in human remyelinating multiple sclerosis plaques. Scientific Reports, 9(1), 3606. 10.1038/s41598-019-40342-x - DOI - PMC - PubMed
-
- Dupree, J. L., Paez, P. M., Tiwari-Woodruff, S. K., Denton, T. T., Hensley, K., Angeliu, C. G., Boullerne, A. I., Kalinin, S., Egge, S., Cheli, V. T., Denaroso, G., Atkinson, K. C., Feri, M., & Feinstein, D. L. (2022). Lanthionine ketimine ethyl ester accelerates remyelination in a mouse model of multiple sclerosis. ASN Neuro, 14(1), 17590914221112352. 10.1177/17590914221112352 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
