2024 Update of Chinese Guidelines for Diagnosis and Treatment of Hyperuricemia and Gout Part I: Recommendations for General Patients
- PMID: 40692263
- PMCID: PMC12280528
- DOI: 10.1111/1756-185x.70375
2024 Update of Chinese Guidelines for Diagnosis and Treatment of Hyperuricemia and Gout Part I: Recommendations for General Patients
Abstract
Background: In 2018, the Chinese Society of Endocrinology developed the "Chinese guideline for diagnosis and treatment of hyperuricemia and gout (2019)". Over the past 5 years, clinical and experimental research has expanded our knowledge of gout, resulting in novel diagnostic and therapeutic approaches. This update, prompted by new clinical challenges and gaps in evidence, aims to refine the 2019 guidelines.
Methods: The working group formulated clinical questions based on a nationwide questionnaire survey, and the expert panel evaluated new evidence addressing these questions from January 2019 to March 2025. The guideline development followed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach, adhering to internationally recognized protocols for clinical practice guideline development.
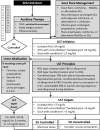
Results: The update includes 26 recommendations addressing 10 clinical questions related to urate-lowering therapy (ULT) for asymptomatic hyperuricemia and reproductive populations, anti-inflammatory treatments, urine alkalinization, dietary advice, and gout diagnosis in patients without a record of hyperuricemia and gout flare predictions in patients with asymptomatic hyperuricemia and intermittent gout. It recommends febuxostat as a first-line ULT for asymptomatic hyperuricemia and using it with caution during pregnancy and lactation. ULT should be customized according to the pathophysiologic type of hyperuricemia. Chronic gout management includes maintaining serum urate levels between 180 and 300 μmol/L and prolonged glucocorticoid tapering in combination with colchicine. Alkalinization with citrate is preferred over sodium bicarbonate for patients with urine pH < 6.0. Novel biomarkers for predicting gout flares are proposed for high-risk populations.
Conclusions: These updated guidelines incorporate expert consensus and evidence to provide refined strategies for the diagnosis, prevention, and treatment of hyperuricemia and gout.
Keywords: diagnosis; gout; guideline; hyperuricemia; management; prediction.
© 2025 The Author(s). International Journal of Rheumatic Diseases published by Asia Pacific League of Associations for Rheumatology and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Richette P., Doherty M., Pascual E., et al., “2018 Updated European League Against Rheumatism Evidence‐Based Recommendations for the Diagnosis of Gout,” Annals of the Rheumatic Diseases 79, no. 1 (2020): 31–38. - PubMed
-
- FitzGerald J. D., Dalbeth N., Mikuls T., et al., “2020 American College of Rheumatology Guideline for the Management of Gout,” Arthritis and Rheumatology 72, no. 6 (2020): 879–895. - PubMed
-
- Chinese Medical Association Chinese Society of Endocrinology , “Guideline for The Diagnosis and Treatment of Hyperuricemia and Gout in China (2019),” Chinese Journal of Endocrinology and Metabolism 36, no. 1 (2020): 13, https://rs.yiigle.com/cmaid/1179377.
-
- Lorenzo J. P. P., Sollano M., Salido E. O., et al., “2021 Asia‐Pacific League of Associations for Rheumatology Clinical Practice Guideline for Treatment of Gout,” International Journal of Rheumatic Diseases 25, no. 1 (2022): 7–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

