Development and Validation of a Rapid LC-MS/MS Method for Plasma Analysis of Ketamine, Norketamine, Dehydronorketamine, and Hydroxynorketamine
- PMID: 40692288
- PMCID: PMC12280840
- DOI: 10.1002/bmc.70171
Development and Validation of a Rapid LC-MS/MS Method for Plasma Analysis of Ketamine, Norketamine, Dehydronorketamine, and Hydroxynorketamine
Abstract
Ketamine, a well-established dissociative anesthetic, has recently gained significant attention for its rapid-acting antidepressant effects, particularly in treatment-resistant depression. In this study, we developed and validated a state-of-the-art liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for the bioanalysis of ketamine and its metabolites, norketamine, dehydronorketamine (DHNK), and (2R,6R)-hydroxynorketamine (HNK), in human plasma. The method features a small sample volume, a streamlined protein precipitation protocol, and a rapid sample runtime. The mobile phase gradient is composed of an aqueous ammonium hydrogen carbonate solution and pure acetonitrile. Using positive electrospray ionization, linear quantification ranges of 1-1,000 ng/mL were established for ketamine and norketamine, while ranges of 0.25-100 ng/mL for DHNK and 2.5-1,000 ng/mL for (2R,6R)-HNK were achieved. The method demonstrated high accuracy, precision, selectivity, and sensitivity, along with consistent matrix effects, efficient extraction recovery, and analyte stability. Finally, the method was successfully applied to assess the pharmacokinetics of six clinical trial participants. Overall, this LC-MS/MS method offers a robust and efficient approach for the achiral quantification of ketamine and its metabolites in human plasma. Its minimal sample preparation and reduced analytical runtime make it particularly well-suited for clinical studies, drug monitoring, and forensic investigations.
Keywords: bioanalysis; clinical study; liquid chromatography; mass spectrometry; pharmacokinetics.
© 2025 The Author(s). Biomedical Chromatography published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
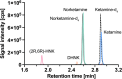
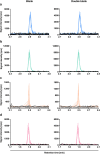
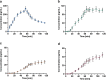
References
-
- Harun, N. , Anderson R. A., and Cormack P. A.. 2010. “Analysis of Ketamine and Norketamine in Hair Samples Using Molecularly Imprinted Solid‐Phase Extraction (MISPE) and Liquid Chromatography‐Tandem Mass Spectrometry (LC‐MS/MS).” Analytical and Bioanalytical Chemistry 396, no. 7: 2449–2459. 10.1007/s00216-009-3404-6. - DOI - PubMed
-
- Hasan, M. , Hofstetter R., Fassauer G. M., Link A., Siegmund W., and Oswald S.. 2017. “Quantitative Chiral and Achiral Determination of Ketamine and Its Metabolites by LC‐MS/MS in Human Serum, Urine and Fecal Samples.” Journal of Pharmaceutical and Biomedical Analysis 139: 87–97. 10.1016/j.jpba.2017.02.035. - DOI - PubMed
-
- Hijazi, Y. , Bolon M., and Boulieu R.. 2001. “Stability of Ketamine and Its Metabolites Norketamine and Dehydronorketamine in Human Biological Samples.” Clinical Chemistry 47, no. 9: 1713–1715. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

