Ligand Type Guided Keto-Arylation Enables Modular Total Synthesis of Polycyclic CBS Xanthones
- PMID: 40692387
- PMCID: PMC12416469
- DOI: 10.1002/anie.202513532
Ligand Type Guided Keto-Arylation Enables Modular Total Synthesis of Polycyclic CBS Xanthones
Abstract
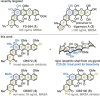
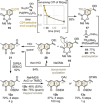
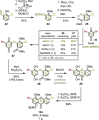
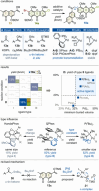
The first total synthesis of the potent polycyclic xanthone antibiotics CBS72, CBS87 and CBS100 was accomplished by a modular strategy featuring a very demanding intermolecular aromatic keto-arylation. Central to the solution was a recently-developed ligand type approach, rather than brute force screening, demonstrating the usefulness of this novel concept in complex target synthesis. Additional key features include an asymmetric Davis hydroxylation proceeding with only catalytic amounts of base, thus enabling the conversion of a highly sensitive, elaborate substrate. Furthermore, a late-stage aminolysis completed the polycyclic framework, circumventing laborious protective group chemistry. Together, this strategy provides a concise, high-yielding access, confirming the full architecture of this most potent class of polyaromatic xanthones, and establishes ligand types as a powerful design tool for sophisticated cross-couplings.
Keywords: Davis oxidation; Keto‐arylation; Ligand types; Polycyclic xanthones; Total synthesis.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Kong L., Deng Z., You D., Nat. Prod. Rep. 2022, 39, 2057. - PubMed
-
- Xie T., Zheng C., Chen K., He H., Gao S., Angew. Chem. Int. Ed. 2020, 59, 4360–4364. - PubMed
-
- Ma A.‐J., Ready J. M., Org. Lett. 2019, 21, 1148–1151. - PubMed
-
- Masuo R., Ohmori K., Hintermann L., Yoshida S., Suzuki K., Angew. Chem. Int. Ed. 2009, 48, 3462–3465. - PubMed
-
- Duthaler R. O., Mathies P., Petter W., Heuberger C., Scherrer V., Helv. Chim. Acta 1984, 67, 1217–1221.
Grants and funding
LinkOut - more resources
Full Text Sources

