Blackberry-Like Doxorubicin Loaded Hyaluronic Acid/Zinc Phthalocyanine Loaded Mesoporous Silica Nanocomposites for Long-Term Tumor Photodynamic and Chemotherapy Synergistic Therapy
- PMID: 40692537
- PMCID: PMC12278960
- DOI: 10.2147/IJN.S530041
Blackberry-Like Doxorubicin Loaded Hyaluronic Acid/Zinc Phthalocyanine Loaded Mesoporous Silica Nanocomposites for Long-Term Tumor Photodynamic and Chemotherapy Synergistic Therapy
Abstract
Background: The co-loading of zinc phthalocyanine (ZnPc) and doxorubicin (DOX) on a nanocarrier for tumor photodynamic therapy (PDT)-chemotherapy (CT) synergistic therapy is an effective approach. However, significant differences in water solubility between DOX and ZnPc hinder their high drug-loading content within a unified carrier. Additionally, DOX's systemic toxicity limits its therapeutic dosage, while low ZnPc loading shortens PDT duration, collectively restricting the efficacy of PDT and related synergistic therapy. This study aims to design a long-term PDT and CT synergistic therapy strategy to significantly improve the therapeutic effect and reduce the toxic side effects.
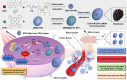
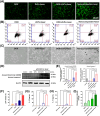
Methods: We encapsulated ZnPc within biodegradable mesoporous silica nanoparticles (bMSN NPs) as the core, followed by electrostatic coating with tumor-targeting, DOX-loaded hyaluronic acid nanoparticles (DOX-HA NPs) to fabricate blackberry-like nanocomposites (DOX-HA/ZnPc-bMSN). In vitro and in vivo experiments determined tumor long-term PDT and CT synergistic therapy efficacy with DOX-HA/ZnPc-bMSN.
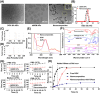
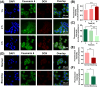
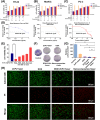
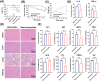
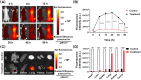
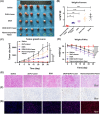
Results: These nanocomposites achieved high ZnPc loading (DLC: 10.2% ± 1.6%) and efficient tumor accumulation, enabling prolonged systemic circulation (> 96 h) and sustained dual-drug release in vivo, realizing long-term photodynamic and CT synergistic therapy. In vitro studies showed a low combination index (CI = 0.26), with reactive oxygen species (ROS) production enhanced by 1.6-fold and 1.9-fold for ZnPc and DOX. The median lethal dose (LD50) of DOX-HA/ZnPc-bMSN nanocomposites (138.95 mg/kg) was 15.12 times higher than that of free DOX. Notably, in vivo studies demonstrated a 96.0% tumor inhibition rate has been achieved using ultralow doses of drugs (DOX: 0.2 mg/kg; ZnPc: 2 mg/kg). This long-term PDT and CT synergistic therapy elevated intracellular ROS levels, which not only induced apoptosis in tumor cells but also activated caspase-1, leading to direct GSDMD cleavage, GSDMD-N release, and pyroptotic tumor cell death.
Conclusion: These nanocomposites dually trigger tumor cell apoptosis/pyroptosis, demonstrating potent therapeutic efficacy and safety for clinical translation.
Keywords: blackberry-like nanocomposites; chemotherapy; mesoporous silica nanoparticles; photodynamic therapy; synergistic therapy.
© 2025 Du et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Polymeric micelles encapsulating pH-responsive doxorubicin prodrug and glutathione-activated zinc(II) phthalocyanine for combined chemotherapy and photodynamic therapy.J Control Release. 2018 Jul 28;282:46-61. doi: 10.1016/j.jconrel.2018.04.030. Epub 2018 Apr 16. J Control Release. 2018. PMID: 29673646
-
An Enzyme-responsive Porphyrin Metal-organic Framework Nanosystem for Targeted and Enhanced Synergistic Cancer Photo-chemo Therapy.Curr Drug Deliv. 2025;22(6):784-797. doi: 10.2174/0115672018286563240223072702. Curr Drug Deliv. 2025. PMID: 38424427
-
2D and 3D in vitro photodynamic activities of tetra-substituted symmetric water-soluble cationic zinc(II) phthalocyanines on cancer.Sci Rep. 2025 Jul 11;15(1):25148. doi: 10.1038/s41598-025-09630-7. Sci Rep. 2025. PMID: 40646060 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Interventions for central serous chorioretinopathy: a network meta-analysis.Cochrane Database Syst Rev. 2025 Jun 16;6(6):CD011841. doi: 10.1002/14651858.CD011841.pub3. Cochrane Database Syst Rev. 2025. PMID: 40522203
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

