Novel potential neuroprotective targets for DengZhanXiXin injection in middle cerebral artery occlusion rats recommended by quantitative proteomics and simulated docking
- PMID: 40692577
- PMCID: PMC12277316
- DOI: 10.3389/fnins.2025.1499214
Novel potential neuroprotective targets for DengZhanXiXin injection in middle cerebral artery occlusion rats recommended by quantitative proteomics and simulated docking
Abstract
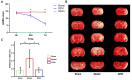
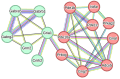
Stroke, which leads to death and disability in high proportions globally, is one of the most deleterious neurological diseases. Ischemic stroke (IS) is the major cause of disease attack and accounts for ~70% of all incident stroke cases in China. Up to now, only two therapies for IS were officially approved, which are intravenous administration of recombinant tissue-plasminogen activator (rt-PA) and endovascular mechanical thrombectomy to rapidly recanalize the occluded artery, which both recanalize the occluded artery rapidly to reduce disability, but are limited in a fixed time window. In this study, the therapeutic effect of a traditional Chinese medicine, DengZhanXiXin injection (DZXI), was evaluated on middle cerebral artery occlusion (MCAO) rats at the neurobehavioral and pathophysiological levels through neurological tests, neurohistological staining, proteomic assay, and biological information analysis. We found that DZXI significantly ameliorated the neurological deficit, prevented infarct volume evolution, and protected cortical neural cells from death in ischemia penumbra on MCAO rats. Furthermore, corresponding therapeutic molecular targets were investigated through proteomic analysis of ischemic hemispheres of MCAO rats. One hundred ninety-one differentially expressed proteins involved in response to metal ions, neurofilament bundle assembly, and modulation of chemical synaptic transmission were identified between the MCAO model and DZXI groups after 7 days. DZXI influenced the expression levels of proteins in 13 specific biological functions, with cell signaling and chemical synaptic transmission-associated proteins being most affected. Subsequent molecular docking analysis predicted binding potential between key target proteins and DZXI compounds. The results suggested that DZXI ameliorates neurological deficits by potentially affecting cellular signaling and chemical synaptic transmission physiological processes.
Keywords: DengZhanXiXin injection; MCAO; cell signaling; ischemic stroke; molecular docking.
Copyright © 2025 Li, Wang, An, Li, Chen, Wei and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Thrombolysis for acute ischaemic stroke.Cochrane Database Syst Rev. 2003;(3):CD000213. doi: 10.1002/14651858.CD000213. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2009 Oct 07;(4):CD000213. doi: 10.1002/14651858.CD000213.pub2. PMID: 12917889 Updated.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- An H., Tao W., Liang Y., Li P., Li M., Zhang X., et al. (2021). Dengzhanxixin injection ameliorates cognitive impairment through a neuroprotective mechanism based on mitochondrial preservation in patients with acute ischemic stroke. Front. Pharmacol. 12:712436. 10.3389/fphar.2021.712436 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

