Investigating Exogenous Tyrosine Supplements on the Responses of the Kale Plant to Salinity Stress
- PMID: 40692608
- PMCID: PMC12277235
- DOI: 10.1002/fsn3.70660
Investigating Exogenous Tyrosine Supplements on the Responses of the Kale Plant to Salinity Stress
Erratum in
-
Correction to "Investigating Exogenous Tyrosine Supplements on the Responses of the Kale Plant to Salinity Stress".Food Sci Nutr. 2025 Aug 27;13(9):e70873. doi: 10.1002/fsn3.70873. eCollection 2025 Sep. Food Sci Nutr. 2025. PMID: 40895145 Free PMC article.
Abstract
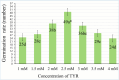
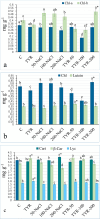
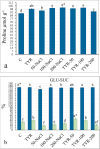
The study investigated the role of exogenous tyrosine (TYR) supplements in extending kale tolerance to NaCl stress at various concentrations (50, 100, and 200 mM). The salt stress was induced by irrigating the soil with salt water, and TYR was supplied through foliar spraying. The impact of TYR supplementation under NaCl stress was assessed by evaluating growth parameters, enzymatic and non-enzymatic defense, oxidative stress markers, and mineral composition. The results revealed that TYR significantly increased the levels of β-carotene, lycopene, anthocyanins, and polyphenols as well as the activities of polyphenol oxidase (PPO), catalase (CAT), and superoxide dismutase (SOD). TYR enhanced the activities of ascorbate peroxidase, CAT, and SOD under 200-NaCl, increased PPO activity at all NaCl concentrations, and reduced the MDA content only at 200-NaCl. The Mg, P, K, Ca, Na, and the ratio K/Na increased under 200-NaCl, while Ca, Na, and Cl declined with lower NaCl. TYR raised Ca and Na levels at 100-NaCl but decreased Na, Cl, and the Na/K ratio at 200-NaCl. In conclusion, high NaCl levels suppressed Chl-a, β-carotene, lycopene, sucrose accumulation, and the activities of PPO, APX, CAT, and SOD, which led to reduced leaf, shoot, and root growth; however, these negative impacts were alleviated by TYR supplementation. The study suggests that to promote agricultural sustainability, it may be advisable to extend tolerance thresholds for moderately tolerant crops, enhance the tolerance of salt-sensitive vegetables in saline regions, and incorporate exogenous TYR.
Keywords: antioxidants; nutrition; sustainability; tolerance.
© 2025 The Author(s). Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Melatonin-Producing Bacillus aerius EH2-5 Enhances Glycine max Plants Salinity Tolerance Through Physiological, Biochemical, and Molecular Modulation.Int J Mol Sci. 2025 Aug 13;26(16):7834. doi: 10.3390/ijms26167834. Int J Mol Sci. 2025. PMID: 40869153 Free PMC article.
-
Physiological and biochemical alterations in soybean by banana peel biochar under different degrees of salt stress.Sci Rep. 2025 Aug 20;15(1):30532. doi: 10.1038/s41598-025-98701-w. Sci Rep. 2025. PMID: 40835709 Free PMC article.
-
Regulation of APX, SOD, and PAL genes by chitosan under salt stress in rapeseed (Brassica napus L.).BMC Plant Biol. 2025 Jul 2;25(1):824. doi: 10.1186/s12870-025-06815-0. BMC Plant Biol. 2025. PMID: 40604426 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Promotion of Ca2+ Accumulation in Roots by Exogenous Brassinosteroids as a Key Mechanism for Their Enhancement of Plant Salt Tolerance: A Meta-Analysis and Systematic Review.Int J Mol Sci. 2023 Nov 9;24(22):16123. doi: 10.3390/ijms242216123. Int J Mol Sci. 2023. PMID: 38003311 Free PMC article.
References
-
- Abdallah, M. M. S. , and El‐Bassiouny H. M. S.. 2016. “Impact of Exogenous Proline or Tyrosine on Growth, Some Biochemical Aspects and Yield Components of Quinoa Plant Grown in Sandy Soil.” International Journal of PharmTech Research 9, no. 7: 12–23.
-
- Abdelkader, M. , Voronina L., Shelepova O., et al. 2023. “Monitoring Role of Exogenous Amino Acids on the Proteinogenic and Ionic Responses of Lettuce Plants Under Salinity Stress Conditions.” Horticulturae 9: 626. 10.3390/horticulturae9060626. - DOI
-
- Alasvandyaria, F. , and Mahdavia B.. 2018. “Effect of Glycine Betaine and Salinity on Photosynthetic Pigments and Ion Concentration of Safflower.” Desert 23, no. 2: 265–271.
-
- Alfosea‐Simon, M. , Simon‐Grao S., Zavala‐Gonzalez E. A., et al. 2020. “Application of Biostimulants Containing Amino Acids to Tomatoes Could Favor Sustainable Cultivation: Implications for Tyrosine, Lysine, and Methionine.” Sustainability 12: 9729.
-
- Al‐Mohammad, M. H. S. , and Al‐Taey D. K. A.. 2019. “Effect of Tyrosine and Sulfur on Growth, Yield and Antioxidant Compounds in Arugula Leaves and Seeds.” Research on Crops 20, no. 1: 116–120. 10.31830/2348-7542.2019.016. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

