High resolution diffusion imaging in the unfixed post-mortem infant brain at 7T
- PMID: 40692625
- PMCID: PMC12043270
- DOI: 10.1162/imag_a_00069
High resolution diffusion imaging in the unfixed post-mortem infant brain at 7T
Abstract
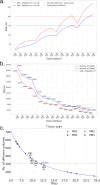
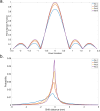
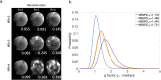
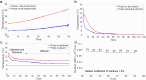
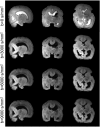
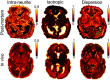
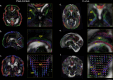
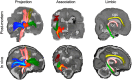
Diffusion MRI of the infant brain allows investigation of the organizational structure of maturing fibers during brain development. Post-mortem imaging has the potential to achieve high resolution by using long scan times, enabling precise assessment of small structures. Technical development for post-mortem diffusion MRI has primarily focused on scanning of fixed tissue, which is robust to effects like temperature drift that can cause unfixed tissue to degrade. The ability to scan unfixed tissue in the intact body would enable post-mortem studies without organ donation, but poses new technical challenges. This paper describes our approach to scan setup, protocol optimization, and tissue protection in the context of the Developing Human Connectome Project (dHCP) of neonates. A major consideration was the need to preserve the integrity of unfixed tissue during scanning in light of energy deposition at ultra-high magnetic field strength. We present results from one of the first two subjects recruited to the study, who died on postnatal day 46 at 29+6 weeks postmenstrual age, demonstrating high-quality diffusion MRI data. We find altered diffusion properties consistent with post-mortem changes reported previously. Preliminary voxel-wise and tractography analyses are presented with comparison to age-matched in vivo dHCP data. These results show that high-quality, high-resolution post-mortem data of unfixed tissue can be acquired to explore the developing human brain.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
