Decoding sex differences in human immunity through systems immunology
- PMID: 40692743
- PMCID: PMC12279299
- DOI: 10.1093/oxfimm/iqaf006
Decoding sex differences in human immunity through systems immunology
Abstract
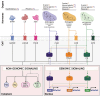
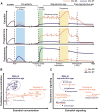
Immune function varies widely across humans. Biological sex is a key factor underlying human immune variability, with men presenting with more severe infections and increased cancer rates, while women exhibit higher vaccine responses and prevalence of autoimmunity. Intrinsic biological sex differences arise from varying contributions of chromosomal sex, and sex hormone sensing and downstream signaling to different cell types. This complex regulation presents a unique opportunity for the exploration of human immune sex differences using systems-level methods of investigation. Here we analyze the current literature and the applications of systems immunology in elucidating the immune sex differences in humans. We examine mechanisms of biological sex modulation of human immunity via sex chromosomes, and particularly emphasize the role of sex hormones. We then focus on how systems immunology has been advancing our understanding of how sex impacts the healthy immune system at steady state, ranging from cell composition, transcriptomics, epigenomics, metabolomics, spatial and cell-cell interactions, to plasma proteomics. We also examine systems-level applications to investigating sex differences upon immune perturbations and give an overview of key future directions for the field. Systems immunology provides a powerful framework to decode biological sex-regulated pathways in immunity, paving the way for more precise, sex-informed therapeutic interventions to address sex differences in immune-related conditions.
Keywords: biological sex; sex differences in human immunity; sex hormones; systems immunology.
© The Author(s) 2025. Published by Oxford University Press.
Figures

Similar articles
-
Understanding patient pathways to Mother and Baby Units: a longitudinal retrospective service evaluation in the UK.Health Soc Care Deliv Res. 2025 Jul 16:1-17. doi: 10.3310/GDVS2427. Online ahead of print. Health Soc Care Deliv Res. 2025. PMID: 40682791
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
-
An Occupational Science Contribution to Camouflaging Scholarship: Centering Intersectional Experiences of Occupational Disruptions.Autism Adulthood. 2025 May 28;7(3):238-248. doi: 10.1089/aut.2023.0070. eCollection 2025 Jun. Autism Adulthood. 2025. PMID: 40539209
-
Fabricating mice and dementia: opening up relations in multi-species research.In: Jenkins N, Jack-Waugh A, Ritchie L, editors. Multi-Species Dementia Studies. Bristol (UK): Bristol University Press; 2025 Feb 25. Chapter 2. In: Jenkins N, Jack-Waugh A, Ritchie L, editors. Multi-Species Dementia Studies. Bristol (UK): Bristol University Press; 2025 Feb 25. Chapter 2. PMID: 40690569 Free Books & Documents. Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
References
Publication types
LinkOut - more resources
Full Text Sources
