The vascular endothelium as decision maker in lung injury
- PMID: 40692751
- PMCID: PMC12277298
- DOI: 10.3389/fcell.2025.1564627
The vascular endothelium as decision maker in lung injury
Abstract
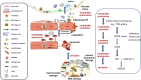
The vascular endothelium is the largest organ in the human body, capable of performing a wide range of cellular signaling and synthetic functions. It is also subjected to considerable mechanical stress due to the shear forces generated by blood flow, which amounts to approximately 10,000 L per day. The endothelial layer plays a crucial role in regulating vascular tone locally and controlling the extravasation of certain blood components. Additionally, it is integral to the coagulation process. The endothelium serves as the entry point for immune cells, which migrate from the bloodstream to surrounding tissues by passing through the endothelial layer. This underscores the importance of proper endothelial function for the health of the body's tissues and organs. When the endothelium fails to perform these functions adequately, leading to endothelial dysfunction, pathological conditions are more likely to develop. Notably, acute lung injury and its severe form, acute respiratory distress syndrome (ARDS), are often associated with endothelial dysfunction. ARDS refers to pulmonary edema with increased vascular permeability resulting from various pulmonary or systemic insults. In most cases, an exaggerated inflammatory and pro-thrombotic response to the initial insult causes disruption of the alveolar-capillary membrane and leakage of vascular fluid. This review emphasizes the central role of the vascular endothelium in acute conditions and seeks to clarify the concepts and interplay between endothelial activation, dysfunction, and damage.
Keywords: damage; endothelial activation; inflammation; lung injury; pulmonary endothelium; vascular dysfunction.
Copyright © 2025 Klein.
Conflict of interest statement
The author declares that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures
Similar articles
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
Ventilator Management(Archived).2023 Mar 27. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2023 Mar 27. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 28846232 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
References
-
- Aird W. C. (2007). Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circulation Res. 100, 174–190. 10.1161/01.RES.0000255690.03436.ae - DOI - PubMed
-
- Alexander Y., Osto E., Schmidt-Trucksäss A., Shechter M., Trifunovic D., Duncker D. J., et al. (2021). Endothelial function in cardiovascular medicine: a consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc Res. 117, 29–42. 10.1093/cvr/cvaa085 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

