Wnt-dependent mechanism of the apical constriction of roof plate cells in developing mouse spinal cord
- PMID: 40692752
- PMCID: PMC12277275
- DOI: 10.3389/fcell.2025.1571770
Wnt-dependent mechanism of the apical constriction of roof plate cells in developing mouse spinal cord
Abstract
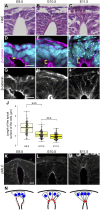
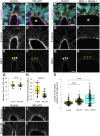
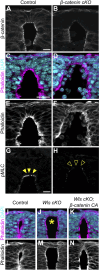
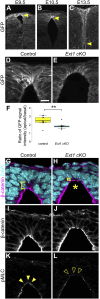
Apical constriction of epithelial cells usually occurs in a local portion of epithelial sheet, which results in bending of epithelial tissues. However, it is uncertain whether diffusible signal molecules, like Wnt, regulate such locally restricted events. Here, we show that Wnt ligands are required for apical constriction of Wnt1-expressing roof plate (RP) cells during development of the neural tube. Analysis of Wntless conditional knock-out (cKO) embryos, in which Wnt secretion from Wnt1-expressing roof plate cells is impaired, revealed that RP-derived Wnt ligands are required for phosphorylation of myosin light chain (MLC) and apical constriction of RP cells. Loss- or gain-of-function analysis of β-catenin reveals that this apical constriction is regulated in a β-catenin-dependent manner. Consistent with the timing of apical constriction, Wnt ligands accumulate on the apical side of RP cells. In embryos with Wnt1-expressing RP-specific defects in synthesis of heparan sulfate proteoglycan, apical accumulation of Wnt ligands and apical constriction are impaired. Therefore, we propose that specific accumulation of Wnt ligands on RP cells drives apical constriction of these cells.
Keywords: Wnt; apical constriction; cytoskeleton; morphogenesis; neural tube; roof plate.
Copyright © 2025 Shinozuka, Okubo, Sasai and Takada.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
Folic acid modifies the shape of epithelial cells during morphogenesis via a Folr1 and MLCK dependent mechanism.Biol Open. 2019 Jan 22;8(1):bio041160. doi: 10.1242/bio.041160. Biol Open. 2019. PMID: 30670450 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Downregulation of Dickkopf-3, a Wnt antagonist elevated in Alzheimer's disease, restores synapse integrity and memory in a disease mouse model.Elife. 2024 Jan 29;12:RP89453. doi: 10.7554/eLife.89453. Elife. 2024. PMID: 38285009 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Vitamin A and fish oils for retinitis pigmentosa.Cochrane Database Syst Rev. 2013 Dec 19;2013(12):CD008428. doi: 10.1002/14651858.CD008428.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2020 Jun 18;6(6):CD008428. doi: 10.1002/14651858.CD008428.pub3. PMID: 24357340 Free PMC article. Updated.
References
-
- Brault V., Moore R., Kutsch S., Ishibashi M., Rowitch D. H., McMahon A. P., et al. (2001). Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253–1264. 10.1242/dev.128.8.1253 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

