R21/Matrix-M malaria vaccine drives diverse immune responses in pre-exposed adults: insights from a phase IIb controlled human malaria infection trial
- PMID: 40692795
- PMCID: PMC12277344
- DOI: 10.3389/fimmu.2025.1620365
R21/Matrix-M malaria vaccine drives diverse immune responses in pre-exposed adults: insights from a phase IIb controlled human malaria infection trial
Abstract
Introduction: The recently licenced R21/Matrix-M vaccine induces a protective antibody response. In this study, we examined vaccine-induced responses in semi-immune adults in a controlled human malaria infection (CHMI) Phase IIb clinical trial.
Methods: Plasma and peripheral blood mononuclear cells from healthy adult volunteers living in coastal Kenya were analysed following vaccination with R21/Matrix-M (n = 19) and CHMI challenge with Plasmodium falciparum (PfSPZ NF54) sporozoites (n = 17). Humoral immunity was evaluated by quantifying antigen specific antibody subtypes and subclasses via ELISA, alongside functional antibody properties including avidity and complement fixation elicited by vaccination and challenge. Antigen-specific memory B cells were characterised using FluoroSpot assays to detect concurrent secretion of multiple antibody isotypes and the frequency and phenotypes of circulating Tfh (cTfh) cells were assessed using multiparametric flow cytometry.
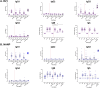
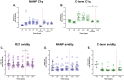
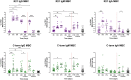
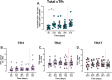
Results: Vaccination increased antibody titres across IgA, IgM, and IgG isotypes and IgG1 and IgG3 subclasses but not IgG2 or IgG4 subclasses, targeting different vaccine antigens (full-length R21, NANP, and C-terminus), indicating a broad and heterogeneous response. The responses were maintained over time and, importantly, they demonstrated complement-fixing capabilities. IgG+ and IgA+ antigen-specific memory B cells were boosted but were short-lived for IgA. We observed an increase in total CXCR5+/PD1+ cTfh cells following vaccination and challenge with the predominant Th2/Th17 population.
Discussion: We provide insights into the diverse immune responses induced by R21/Matrix-M vaccination and their potential contribution to protection against malaria. These findings highlight the potential of the R21/Matrix-M vaccination and protection in adults with varying levels of prior malaria exposure.
Keywords: R21/Matrix-M; T follicular helper cells; antibodies; complement fixing antibodies; controlled human malaria infection; malaria; memory B cells.
Copyright © 2025 Kibwana, Bundi, Kimani, Nyamako, Keter, Mutiso, Ogwang, Bellamy, Rapi, Bajer, Provstgaard-Morys, Stockdale, Munoz, Datoo, Lawrie, Ramos-Lopez, Roberts, Hamaluba, Hill, Bejon, Ewer and Kapulu.
Conflict of interest statement
AH and KE are co-inventors of patent applications related to R21. KE was an employee of the University of Oxford at the time of the work and is now an employee of GSK and owns restricted shares in GSK. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- WHO . World malaria report(2024). Available online at: https://www.wipo.int/amc/en/mediation/%0Ahttps://www.who.int/teams/globa... (Accessed January 12, 2025).
-
- WHO. World Health Organization . WHO recommends R21/Matrix-M vaccine for malaria prevention in updated advice on immunization(2023). Available online at: https://www.who.int/news/item/02-10-2023-who-recommends-r21-matrix-m-vac... (Accessed December 4, 2023).
-
- Sang S, Datoo M S, Otieno E, Muiruri C, Bellamy D, Gathuri E, et al. Safety and immunogenicity of varied doses of R21/Matrix-M™ vaccine at three years follow-up: A phase 1b age de-escalation, dose-escalation trial in adults, children, and infants in Kilifi-Kenya. Wellcome Open Res. (2023) 8:450. doi: 10.12688/wellcomeopenres, PMID: - DOI - PMC - PubMed
-
- Datoo MS, Dicko A, Tinto H, Ouédraogo JB, Hamaluba M, Olotu A, et al. Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: a multicentre, double- blind, randomised, phase 3 trial. Lancet. (2024) 403(10426):533–44. doi: 10.1016/S0140-6736(23)02511-4, PMID: - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

