Effects of a polyphenol-rich extract blend, probiotics, and hydrolyzed fiber on quality of life and gut health markers in patients with irritable bowel syndrome-A randomized, double-blind, placebo-controlled trial
- PMID: 40693198
- PMCID: PMC12277158
- DOI: 10.3389/fnut.2025.1603011
Effects of a polyphenol-rich extract blend, probiotics, and hydrolyzed fiber on quality of life and gut health markers in patients with irritable bowel syndrome-A randomized, double-blind, placebo-controlled trial
Abstract
Introduction: Irritable bowel syndrome (IBS) is the most prevalent functional bowel disorder impacting around 5%-10% of the general population worldwide. The pathogenesis remains unclear, however alterations in gut-brain axis play a critical role. We aimed to investigate the therapeutic potential of a novel synbiotic formulation comprising of partially hydrolyzed guar gum (PHGG), specific probiotic strains (Bifidobacterium and Saccharomyces boulardii), and double-standardized, polyphenol-rich blend of extracts from Aronia melanocarpa and Sambucus nigra in patients with IBS.
Methods: A total of 47 patients with IBS were randomly assigned to three groups and followed over a 2-month study period. Group I (n = 14) received placebo capsules, Group II (n = 14) took one placebo capsule along with a probiotic formulation and PHGG, Group III (n = 19) received probiotic formulation, PHGG and polyphenol-rich fruit extracts blend. The IBS-quality of life (QoL) questionnaire was completed by all participants at baseline and after 2 months. Serum levels of IL-6, IL-8, TNF-α, I-FABP-2, GM-CSF and stool concentrations of short-chain fatty acids (SCFAs) and zonulin were evaluated before and after intervention.
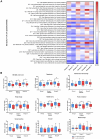
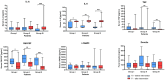
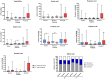
Results and conclusions: This study demonstrated a significant improvement in QoL in individuals receiving the complete formulation combination (Group III). The largest decrease in score was observed in dysphoria, with median differences of -5 in Group III (p = 0.0021), -3 in Group II (p = 0.0155), and -1 in the control Group I (p = 0.0338). Significant correlations were found in Groups II and III between supplementation and serum concentrations of IL-8, TNF, and GM-CSF (p < 0.05). A significantly higher concentration of all SCFAs was seen after intervention in Group III compared to control Group I.
Keywords: FODMAP; SCFAs; dietary intervention; fiber; irritable bowel syndrome; polyphenols; probiotics; quality of life.
Copyright © 2025 Wierzbicka, Khaidakov, Zakerska-Banaszak, Andrzejewska, Baturo, Kowalczyk, Lemke, Dobrowolska, Skrzypczak-Zielinska and Mankowska-Wierzbicka.
Conflict of interest statement
BK, PK and KL have a patent for Fenactive® blend no. 245254 and a patent pending for Fenactive® blend no. P.437487. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Staudacher HM, Whelan K, Irving PM, Lomer MCE. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Human Nutrition Diet. (2011) 24:487–95. 10.1111/j.1365-277X.2011.01162.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

