The human STING agonist E7766 induces immunogenic tumor clearance, independent of tumor-intrinsic STING expression in the KRASG12D/+ Trp53-/- murine model of sarcoma
- PMID: 40693387
- PMCID: PMC12296111
- DOI: 10.1080/2162402X.2025.2534912
The human STING agonist E7766 induces immunogenic tumor clearance, independent of tumor-intrinsic STING expression in the KRASG12D/+ Trp53-/- murine model of sarcoma
Abstract
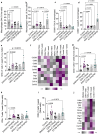
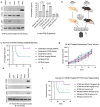
Soft tissue sarcomas (STS) are aggressive high-fatality cancers that affect children and adults. Most STS subtypes harbor an immunosuppressive tumor microenvironment (TME) and respond poorly to immunotherapy. Therapies capable of dismantling the immunosuppressive TME are needed to improve sensitivity to emerging immunotherapies. Activation of the Stimulator of INterferon Genes (STING) pathway has shown promising anti-tumor effects in preclinical models of carcinoma, but evaluations in sarcoma are lacking. Herein, we sought to examine the immune modulation and therapeutic efficacy of three translational small molecule STING agonists in an immunologically cold model of STS. Three classes of STING agonists, ML RR-S2 CDA, MSA-2, and E7766 were evaluated in an orthotopic KrasG12D/+ Trp53-/- model of STS. Dose titration survival studies, cytokine serology, and tumor immune phenotyping were used to examine STING agonist efficacy following intra-tumoral treatment. All STING agonists significantly increased survival time, however, only E7766 resulted in durable tumor clearance, inducing CD8+ T-cell infiltration and activated lymphocyte transcriptomic signatures in the TME. Antibody depletion was used to assess the dependency of treatment responses on CD8+ T-cells, showing that in their absence, tumor clearance did not occur following E7766 therapy. Using STING deficient mice, and CRISPR/Cas9 gene editing, we demonstrated that STS clearance following STING therapy was dependent on host STING and not tumor-intrinsic STING pathway functionality. E7766 represents a promising candidate able to remodel the TME of murine STS tumors toward an inflamed phenotype independent of tumor-intrinsic STING functionality, and should be considered for potential translation in STS treatment.
Keywords: Immunotherapy; STING; Solid tumor; sarcoma.
Conflict of interest statement
The authors of this manuscript do not have any relevant competing interests or conflicts of interest to disclose.
Figures




References
-
- Fletcher C, Bridge JA, Hogendoorn PCW, Mertens F.. WHO classification of tumours of soft tissue and bone: WHO classification of tumours. Vol. 5. IARC, World Health Organization; 2013.
-
- D’Angelo SP, Mahoney MR, Van Tine BA, Atkins J, Milhem MM, Jahagirdar BN, Antonescu CR, Horvath E, Tap WD, Schwartz GK. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018;19(3):416–426. doi: 10.1016/S1470-2045(18)30006-8. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
