Stereospecific Synthesis of Cyclobutanones and Spirohexanones via Formal [3 + 1] Cycloaddition of Cyclopropanones with Sulfur Ylides
- PMID: 40693674
- PMCID: PMC12323705
- DOI: 10.1021/acs.orglett.5c02601
Stereospecific Synthesis of Cyclobutanones and Spirohexanones via Formal [3 + 1] Cycloaddition of Cyclopropanones with Sulfur Ylides
Abstract
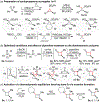
A concise synthetic route to enantioenriched cyclobutanones is reported via ring expansion of cyclopropanone surrogates with unstabilized sulfoxonium ylides. The reaction is shown to proceed with complete regio- and stereospecificity with chiral substrates, leading to optically active 2,3-disubstituted cyclobutanones where reversible enamine formation allowed for controlled equilibration to the thermodynamic trans diastereomer. Alternatively, employing Trost's cyclopropylsulfonium reagent provided the first synthesis of enantioenriched spiro[2.3]hexan-4-ones via a unique semipinacol rearrangement of a dicyclopropyl betaine intermediate.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- For selected reviews related to the synthesis and applications of optically active cyclobutane derivatives, see:
- Lee-Ruff E; Mladenova G Enantiomerically Pure Cyclobutane Derivatives and Their Use in Organic Synthesis. Chem. Rev 2003, 103, 1449–1484. - PubMed
- Secci F; Frongia A; Piras PP Stereocontrolled Synthesis and Functionalization of Cyclobutanes and Cyclobutanones. Molecules 2013, 18, 15541–15572. - PMC - PubMed
- Xu Y; Conner ML; Brown MK Cyclobutane and Cyclobutene Synthesis: Catalytic Enantioselective [2+2] Cycloadditions. Angew. Chem., Int. Ed 2015, 54, 11918–11928. - PubMed
- Poplata S; Tröster A; Zou Y-Q; Bach T Recent Advances in the Synthesis of Cyclobutanes by Olefin [2 + 2] Photocycloaddition Reactions. Chem. Rev 2016, 116, 9748–9815. - PMC - PubMed
- Sietmann J; Wahl JM Enantioselective Desymmetrization of Cyclobutanones: A Speedway to Molecular Complexity. Angew. Chem., Int. Ed 2020, 59, 6964–6974. - PMC - PubMed
- Wang M; Zhong C; Lu P Enantioselective Functionalization of Prochiral Cyclobutanones and Cyclobutenones. Synlett 2021, 32, 1253–1259.
- Barday M; Bouillac P; Coquerel Y; Amatore M; Constantieux T; Rodriguez J Enantioselective Organocatalytic Syntheses and Ring-Expansions of Cyclobutane Derivatives. Eur. J. Org. Chem 2021, 3023–3034.
-
- For selected examples of cyclobutanone one-atom ring expansion processes employed as key steps in the synthesis of complex molecules, see:
- Greene AE; Deprès JP; Nagano H; Crabbé P Ring expansion reactions of 11-nor PGE2. Synthesis of new lactone, lactam and cyclopentanone prostanoids1. Tetrahedron Lett 1977, 18, 2365–2366.
- Trost BM; Balkovec JM; Mao MKT A biomimetic approach to plumericin. J. Am. Chem. Soc 1983, 105, 6755–6757.
- Corey EJ; Kang MC; Desai MC; Ghosh AK; Houpis IN Total synthesis of (±)-ginkgolide B. J. Am. Chem. Soc 1988, 110, 649–651. - PMC - PubMed
- Crimmins MT; Jung DK; Gray JL A total synthesis of (±)-bilobalide. J. Am. Chem. Soc 1992, 114, 5445–5447.
- Wendt JA; Gauvreau PJ; Bach RD Synthesis of (±)-Fredericamycin A. J. Am. Chem. Soc 1994, 116, 9921–9926.
- Meng D; Danishefsky SJ Stereospecific Sulfur-Mediated Cleavage of a Spirocyclobutanone: Synthesis of a Fully Functional Precursor to the CP Compounds. Angew. Chem., Int. Ed 1999, 38, 1485–1488. - PubMed
- Nicolaou KC; Pfefferkorn JA; Kim S; Wei HX Synthesis of the Fully Functionalized Bicyclic Core of Garsubellin A. J. Am. Chem. Soc 1999, 121, 4724–4725.
- Roche C; Kadlečíková K; Veyron A; Delair P; Philouze C; Greene AE; Flot D; Burghammer M New Asymmetric Approach to Natural Pyrrolizidines: Synthesis of (+)-Amphorogynine A, (+)-Amphorogynine D, and (+)-Retronecine. J. Org. Chem 2005, 70, 8352–8363. - PubMed
- Wang Z; Min S-J; Danishefsky SJ Total Synthesis and Structural Revision of (±)-Tricholomalides A and B. J. Am. Chem. Soc 2009, 131, 10848–10849. - PMC - PubMed
- Meyer AM; Katz CE; Li S-W; Vander Velde D; Aubé J A Tandem Prins/Schmidt Reaction Approach to Marine Alkaloids: Formal and Total Syntheses of Lepadiformines A and C. Org. Lett 2010, 12, 1244–1247. - PMC - PubMed
- Liu R; Gutierrez O; Tantillo DJ; Aubé J Stereocontrol in a Combined Allylic Azide Rearrangement and Intramolecular Schmidt Reaction. J. Am. Chem. Soc 2012, 134, 6528–6531. - PMC - PubMed
- Ma A-J; Tu Y-Q; Peng J-B; Dou Q-Y; Hou S-H; Zhang F-M; Wang S-H Total Synthesis of (–)-FR901483. Org. Lett 2012, 14, 3604–3607. - PubMed
- Rasik CM; Brown MK Total Synthesis of Gracilioether F: Development and Application of Lewis Acid Promoted Ketene–Alkene [2+2] Cycloadditions and Late-Stage C–H Oxidation. Angew. Chem., Int. Ed 2014, 53, 14522–14526. - PubMed
- Ruider SA; Carreira EM A Unified Strategy to Plakortin Pentalenes: Total Syntheses of (±)-Gracilioethers E and F. Org. Lett 2016, 18, 220–223. - PubMed
- Rullière P; Cannillo A; Grisel J; Cividino P; Sébastien C; Poisson J-F Total Synthesis of Proteasome Inhibitor (–)-Omuralide through Asymmetric Ketene [2 + 2]-Cycloaddition. Org. Lett 2018, 20, 4558–4561. - PubMed
- Li Q; Zhao K; Peuronen A; Rissanen K; Enders D; Tang Y Enantioselective Total Syntheses of (+)-Hippolachnin A, (+)-Gracilioether A, (–)-Gracilioether E, and (–)-Gracilioether F. J. Am. Chem. Soc 2018, 140, 1937–1944. - PubMed
- Schmid M; Grossmann AS; Wurst K; Magauer T Total Synthesis of Salimabromide: A Tetracyclic Polyketide from a Marine Myxobacterium. J. Am. Chem. Soc 2018, 140, 8444–8447. - PMC - PubMed
- Lin Y; Zhang R; Wang D; Cernak T Computer-aided key step generation in alkaloid total synthesis. Science 2023, 379, 453–457. - PubMed
- Ba M; He F; Ren L; Whittingham WG; Yang P; Li A Scalable Total Synthesis of Acremolactone B. Angew. Chem., Int. Ed 2024, 63, e202314800. - PubMed
-
- For selected reviews on the metal-catalyzed ring expansion of cyclobutanones, see:
- Murakami M; Ishida N Potential of Metal-Catalyzed C–C Single Bond Cleavage for Organic Synthesis. J. Am. Chem. Soc 2016, 138, 13759–13769. - PubMed
- Chen P.-h.; Billett BA; Tsukamoto T; Dong G “Cut and Sew” Transformations via Transition-Metal-Catalyzed Carbon–Carbon Bond Activation. ACS Catal 2017, 7, 1340–1360. - PMC - PubMed
- Deng L; Dong G Carbon‒Carbon Bond Activation of Ketones. Trends Chem 2020, 2, 183–198.
- Murakami M; Ishida N Cleavage of Carbon–Carbon σ-Bonds of Four-Membered Rings. Chem. Rev 2021, 121, 264–299. - PubMed
- Xue Y; Dong G Deconstructive Synthesis of Bridged and Fused Rings via Transition-Metal-Catalyzed “Cut-and-Sew” Reactions of Benzocyclobutenones and Cyclobutanones. Acc. Chem. Res 2022, 55, 2341–2354. - PMC - PubMed
- Cao J; Xu L-W Palladium- and nickel-catalyzed cascade enantioselective ring-opening/coupling reactions of cyclobutanones. Chem. Commun 2023, 59, 3373–3382. - PubMed
-
- For selected examples of C–C activation of cyclobutanones leading to ring contraction instead via decarbonylation, see:
- Murakami M; Amii H; Ito Y Selective activation of carbon–carbon bonds next to a carbonyl group. Nature 1994, 370, 540–541.
- Murakami M; Amii H; Shigeto K; Ito Y Breaking of the C–C Bond of Cyclobutanones by Rhodium(I) and Its Extension to Catalytic Synthetic Reactions. J. Am. Chem. Soc 1996, 118, 8285–8290.
- Zhou X; Ko HM; Dong G Synthesis of Bridged Cyclopentane Derivatives by Catalytic Decarbonylative Cycloaddition of Cyclobutanones and Olefins. Angew. Chem., Int. Ed 2016, 55, 13867–13871. - PMC - PubMed
-
- For selected examples and reviews of metal-catalyzed ring opening of cyclobutanone derivatives, see:
- Matsuda T; Makino M; Murakami M Rhodium-Catalyzed Addition/Ring-Opening Reaction of Arylboronic Acids with Cyclobutanones. Org. Lett 2004, 6, 1257–1259. - PubMed
- Xiao F; Guo Y; Zeng Y-F Recent Developments in Radical Cross-Coupling of Redox-Active Cycloketone Oximes. Adv. Synth. Catal 2021, 363, 120–143.
- Zhang M; Gao J; Zhao J; Qiu T; Li Z; Guo Z; Liu C; Liu Y Lewis Acid Catalyzed Ring-Opening Reaction of Cyclobutanones towards Conjugated Enones. Eur. J. Org. Chem 2021, 6111–6114.
- Ano Y; Takahashi D; Yo K; Nagamune R; Chatani N Palladium-Catalyzed Ring Opening of Cyclobutanones with Carbon- and Heteroatom-Centered Nucleophiles. Synlett 2023, 34, 2486–2490.
- Jiang H-M; Zhao Y-L; Sun Q; Ouyang X-H; Li J-H Recent Advances in N-O Bond Cleavage of Oximes and Hydroxylamines to Construct N-Heterocycle. Molecules 2023, 28, 1775. - PMC - PubMed
- Zhong L-J; Fan J-H; Chen P; Huang P-F; Xiong B-Q; Tang K-W; Liu Y Recent advances in ring-opening of cyclobutanone oximes for capturing SO2, CO or O2 via a radical process. Org. Biomol. Chem 2024, 22, 10–24. - PubMed
- Zhang D-Y; Tao J; Liu Z-Y; Li T-G; Ma W-H; Li T-T; Wu Y; Liu Y Water Participated Dual C(sp3)–C(sp3) Bond Cleavage of Cyclobutanones Catalyzed by Lewis Acid. ChemistrySelect 2024, 9, e202402873.
Grants and funding
LinkOut - more resources
Full Text Sources

