Male Breast Cancer Complicated With Leukocytosis Resembling Leukemia Reaction After Chemotherapy: A Case Report
- PMID: 40693684
- PMCID: PMC12281595
- DOI: 10.1002/cnr2.70280
Male Breast Cancer Complicated With Leukocytosis Resembling Leukemia Reaction After Chemotherapy: A Case Report
Abstract
Introduction: Male breast cancer (MBC) accounts for less than 1% of all cancers in men, with invasive ductal carcinoma being the most common type. The chemotherapy regimens used for MBC are similar to those for female breast cancer. However, the incidence of chemotherapy-induced complications such as leukocytosis resembling leukemia reaction is not well documented in MBC. This case highlights a rare complication in an MBC patient, induced by prophylactic PEG-rhG-CSF following chemotherapy.
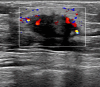
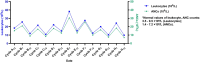
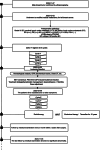
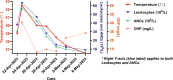
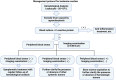
Case presentation: A 51-year-old male with left breast invasive ductal carcinoma underwent modified radical mastectomy. Postoperative pathology revealed high-risk features, and the patient received 8 cycles of chemotherapy with the ddAC-T regimen, followed by PEG-rhG-CSF for febrile neutropenia prevention. After the fifth chemotherapy cycle, the patient developed leukocytosis resembling leukemia reaction, characterized by a white blood cell count exceeding 50 × 109/L, along with intermittent fever up to 42.5°C. The condition was attributed to the PEG-rhG-CSF administration, and the patient was treated with NSAIDs and dexamethasone. Leukocytosis resolved after adjusting the PEG-rhG-CSF dose.
Conclusion: Leukocytosis resembling leukemia reaction induced by PEG-rhG-CSF post-chemotherapy is a rare complication, particularly in MBC patients. This case underscores the importance of careful monitoring and differential diagnosis to avoid misdiagnosis and unnecessary interventions. Personalized treatment strategies and dose adjustments for PEG-rhG-CSF are crucial in managing this rare reaction, emphasizing the need for awareness and individualized care in MBC patients undergoing chemotherapy.
Keywords: chemotherapy; leukocytosis resembling leukemia reaction; male breast cancer.
© 2025 The Author(s). Cancer Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Fox S., Speirs V., and Shaaban A. M., “Male Breast Cancer: An Update,” Virchows Archiv 480, no. 1 (2022): 85–93. - PubMed
-
- Network N C C , “NCCN Clinical Practice Guidelines in Oncology: Breast Cancer (Version 6.2024)[S],” 2024.
-
- Becker P. S., Griffiths E. A., Alwan L. M., et al., “NCCN Guidelines Insights: Hematopoietic Growth Factors, Version 1.2020,” Journal of the National Comprehensive Cancer Network 18, no. 1 (2020): 12–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

