Topical Gel/Microsphere Eyedrop for Combined Delivery of Brimonidine and Timolol: A Comparative Study With Traditional Eye Drops in Rabbits
- PMID: 40693711
- PMCID: PMC12302038
- DOI: 10.1167/tvst.14.7.15
Topical Gel/Microsphere Eyedrop for Combined Delivery of Brimonidine and Timolol: A Comparative Study With Traditional Eye Drops in Rabbits
Abstract
Purpose: Topical eye drops combining multiple antiglaucoma agents are often required when monotherapy fails to reduce intraocular pressure (IOP). However, their effectiveness is compromised by low bioavailability and poor patient compliance. To address these issues, we developed a novel gel/microsphere eye drop (GME) containing brimonidine and timolol, aimed at enhancing ocular bioavailability, reducing dosing frequency, and improving patient compliance.
Methods: The GME system comprises separate formulations of brimonidine-loaded and timolol-loaded polymer microspheres within a thermoresponsive hydrogel, enabling simple off-the-shelf preparation. We examined the in vitro drug release, compared the biodistribution with traditional eye drops, and assessed pharmacodynamic effects in New Zealand white rabbits and Dutch Belted rabbits.
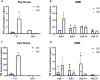
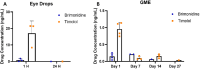
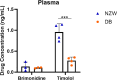
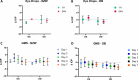
Results: Brimonidine and timolol reached high concentrations in the aqueous and vitreous humor at 1 hour following eye drop administration, declining to nearly undetectable levels by 24 hours. The GME system at a similar dose extended the drug release to 27 days with initially lower drug levels but decreasing more slowly. The GME system also resulted in significantly lower plasma drug concentrations than eye drops, suggesting a reduced risk of systemic side effects. Neither the GME nor eye drops significantly reduced IOP in normotensive rabbits.
Conclusions: The GME system presents a promising alternative to traditional eye drops for controlled drug release in ocular applications, enabling sustained drug delivery while minimizing systemic exposure and thereby potentially enhancing the safety and efficacy of ocular therapies.
Translational relevance: The GME system bridges basic research and clinical application by providing a controlled-release platform that sustains drug delivery, reduces systemic side effects, and enhances patient adherence.
Conflict of interest statement
Disclosure:
Figures



References
-
- Jayaram H, Kolko M, Friedman DS, Gazzard G.. Glaucoma: now and beyond. Lancet. 2023; 402(10414): 1788–1801. - PubMed
-
- Kass MA, Heuer DK, Higginbotham EJ, et al.. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol . 2002; 120(6): 701–713. - PubMed
-
- Kobelt-Nguyen G, Gerdtham U-G, Alm A.. Costs of treating primary open-angle glaucoma and ocular hypertension: a retrospective, observational two-year chart review of newly diagnosed patients in Sweden and the United States. J Glaucoma . 1998; 7(2): 95–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

