A genome-wide association study implicates the olfactory system in Drosophila melanogaster diapause-associated lifespan extension and fecundity
- PMID: 40693912
- PMCID: PMC12283071
- DOI: 10.7554/eLife.98142
A genome-wide association study implicates the olfactory system in Drosophila melanogaster diapause-associated lifespan extension and fecundity
Abstract
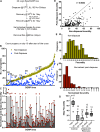
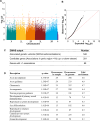
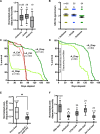
The effects of environmental stress on animal life are gaining importance with climate change. Diapause is a dormancy program that occurs in response to an adverse environment, followed by resumption of development and reproduction upon the return of favorable conditions. Diapause is a complex trait, so we leveraged the Drosophila Genetic Reference Panel (DGRP) lines and conducted a genome-wide association study (GWAS) to characterize the genetic basis of diapause. We assessed post-diapause and non-diapause fecundity across 193 DGRP lines. GWAS revealed 546 genetic variants, encompassing single nucleotide polymorphisms, insertions, and deletions associated with post-diapause fecundity. We identified 291 candidate diapause-associated genes, 40 of which had previously been associated with diapause, and 89 of which were associated with more than one SNP. Gene network analysis indicated that the diapause-associated genes were primarily linked to neuronal and reproductive system development. Similarly, comparison with results from other fly GWAS revealed the greatest overlap with olfactory-behavior-associated and fecundity-and-lifespan-associated genes. An RNAi screen of selected candidates identified two neuronal genes, Dip-γ and Scribbler, to be required during recovery for post-diapause fecundity. We complemented the genetic analysis with a test of which neurons are required for successful diapause. We found that although amputation of the antenna had little to no effect on non-diapause lifespan, it reduced diapause lifespan and post-diapause fecundity. We further show that olfactory receptor neurons and temperature-sensing neurons are required for successful recovery from diapause. Our results provide insights into the molecular, cellular, and genetic basis of adult reproductive diapause in Drosophila.
Keywords: D. melanogaster; diapause; dormancy; fecundity; genetics; genomics; lifespan; resilience; stress.
© 2024, Easwaran and Montell.
Conflict of interest statement
SE, DM No competing interests declared
Figures


Update of
-
A genome-wide association study implicates the olfactory system in Drosophila diapause-associated lifespan extension and fecundity.bioRxiv [Preprint]. 2025 Jun 18:2024.03.10.584341. doi: 10.1101/2024.03.10.584341. bioRxiv. 2025. Update in: Elife. 2025 Jul 22;13:RP98142. doi: 10.7554/eLife.98142. PMID: 39005458 Free PMC article. Updated. Preprint.
Similar articles
-
A genome-wide association study implicates the olfactory system in Drosophila diapause-associated lifespan extension and fecundity.bioRxiv [Preprint]. 2025 Jun 18:2024.03.10.584341. doi: 10.1101/2024.03.10.584341. bioRxiv. 2025. Update in: Elife. 2025 Jul 22;13:RP98142. doi: 10.7554/eLife.98142. PMID: 39005458 Free PMC article. Updated. Preprint.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
Stress-induced Cdk5 activity enhances cytoprotective basal autophagy in Drosophila melanogaster by phosphorylating acinus at serine437.Elife. 2017 Dec 11;6:e30760. doi: 10.7554/eLife.30760. Elife. 2017. PMID: 29227247 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Drosophila seminal sex peptide associates with rival as well as own sperm, providing SP function in polyandrous females.Elife. 2020 Jul 16;9:e58322. doi: 10.7554/eLife.58322. Elife. 2020. PMID: 32672537 Free PMC article.
References
-
- Budelli G, Ni L, Berciu C, van Giesen L, Knecht ZA, Chang EC, Kaminski B, Silbering AF, Samuel A, Klein M, Benton R, Nicastro D, Garrity PA. Ionotropic receptors specify the morphogenesis of phasic sensors controlling rapid thermal preference in Drosophila. Neuron. 2019;101:738–747. doi: 10.1016/j.neuron.2018.12.022. - DOI - PMC - PubMed
-
- Clarke DJB, Jeon M, Stein DJ, Moiseyev N, Kropiwnicki E, Dai C, Xie Z, Wojciechowicz ML, Litz S, Hom J, Evangelista JE, Goldman L, Zhang S, Yoon C, Ahamed T, Bhuiyan S, Cheng M, Karam J, Jagodnik KM, Shu I, Lachmann A, Ayling S, Jenkins SL, Ma’ayan A. Appyters: turning jupyter notebooks into data-driven web apps. Patterns. 2021;2:100213. doi: 10.1016/j.patter.2021.100213. - DOI - PMC - PubMed

