Perspectives on Artificial Intelligence in Medical Publishing: A Survey of Medical Journal Editors
- PMID: 40693971
- PMCID: PMC12499947
- DOI: 10.1097/FJC.0000000000001738
Perspectives on Artificial Intelligence in Medical Publishing: A Survey of Medical Journal Editors
Abstract
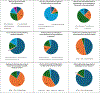
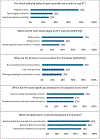
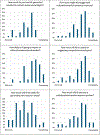
Artificial intelligence (AI) has been increasingly integrated into medical publishing, hopefully improving efficiency and accuracy, but serious concerns persist regarding ethical implications, authorship attribution, and content reliability. We aimed at understanding the perspectives of editors of medical journals on AI. A structured online questionnaire was developed and distributed to editors-in-chief of medical journals worldwide. The survey comprised 27 concise questions exploring demographics, journal practices, and perspectives on AI in editorial workflows. Quantitative data were analyzed using descriptive statistics to summarize usage patterns, perceived benefits, risks, and future expectations. A total of 59 editors-in-chief completed the survey (response rate: 19%), with replies suggesting substantial variability in beliefs and attitudes toward AI for publication in medical journals. Artificial intelligence tools were already in use by 49% of journals, mainly for plagiarism detection (76%) and data verification (35%). Only 9% of responders reported that journals used AI for both scientific and linguistic review. Time savings (79%) and cost reduction (43%) were the most commonly cited benefits, and concerns included potential bias (71%) and lack of accountability (60%). Overall, 81% of responders anticipated a major role for AI in publishing within 10 years. Exploratory analyses suggested several potential associations between replies and respondent or journal features, requiring further validation in future surveys. In conclusion, this survey on attitudes toward AI in publication in medical journals suggests that editors-in-chief are cautiously adopting AI in their editorial workflow, supporting its operational use while explicitly calling for clear guidance to address ethical and regulatory concerns.
Keywords: artificial intelligence; editor; journal; publishing; research; survey.
Copyright © 2025 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
G. Biondi-Zoccai has consulted, lectured, and/or served as advisory board member for Abiomed, Advanced Nanotherapies, Aleph, Amarin, AstraZeneca, Balmed, Cardionovum, Cepton, Crannmedical, Endocore Lab, Eukon, Guidotti, Innovheart, Meditrial, Menarini, Microport, Opsens Medical, Synthesa, Terumo, and Translumina, outside the present work. D. L. Bhatt is in the advisory board of Angiowave, Bayer, Boehringer Ingelheim, CellProthera, Cereno Scientific, E-Star Biotech, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, NirvaMed, Novo Nordisk, Stasys; and Tourmaline Bio is one of the board of directors in American Heart Association New York City; holds stock or stock options in Angiowave, Bristol-Myers Squibb, DRS.LINQ, and High Enroll; is a consultant for Broadview Ventures, Corcept Therapeutics, GlaxoSmithKline, Hims, SFJ, Summa Therapeutics, and Youngene; is in the data monitoring committee of Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic, Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical; for ALLAY-HF, funded by Alleviant Medical), Novartis, Population Health Research Institute; and Rutgers University (for the NIH-funded MINT Trial); reports honoraria from American College of Cardiology (senior associate editor, Clinical Trials and News, ACC.org ; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (editor-in-chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), CSL Behring (AHA lecture), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (editor-in-chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (guest editor; associate editor), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (course director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and US national coleader, funded by Bayer), and WebMD (CME steering committees), Wiley (steering committee); and other relationships as follows: Clinical Cardiology (deputy editor); patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women's Hospital who assigned to Lexicon; neither I nor Brigham and Women's Hospital receive any income from this patent); research funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Alnylam, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, Cleerly, CSL Behring, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Otsuka, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, and 89Bio; and royalties from Elsevier (editor, Braunwald's Heart Disease); and is a site coinvestigator for Cleerly. A. Eggermont has consulted and/or served as advisory board member for Agenus, Boehringer Ingelheim, BioInvent, BioNTech, Brenus, CatalYm, Egle, Eurobio, Imcheck, IO Biotech, IQVIA, Merck&Co, MSD, Oncolytics, Pierre Fabre, Pfizer, QBiotics, Regeneron, Sairopa, Scorpion, SkylineDX, Trained Immunity TX Discovery. Equity: IO Biotech, Sairopa, SkylineDX. All other authors report no conflict of interest.
Figures
References
-
- Biondi-Zoccai G, editor. ChatGPT for Medical Research. Torino: Edizioni Minerva Medica; 2024.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources