Lacticaseibacillus paracasei Glu-07: A Promising Probiotic Candidate for the Management of Type 2 Diabetes Mellitus
- PMID: 40694135
- PMCID: PMC12283894
- DOI: 10.1007/s00284-025-04391-y
Lacticaseibacillus paracasei Glu-07: A Promising Probiotic Candidate for the Management of Type 2 Diabetes Mellitus
Abstract
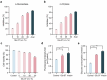
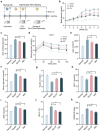
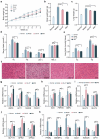
The global prevalence of type 2 diabetes mellitus (T2DM) is currently surging, posing significant health and socioeconomic burdens. Probiotics have emerged as promising interventions due to their safety and potential metabolic benefits. Gut microbiota plays a critical role in the pathophysiology of metabolic diseases in humans, offering valuable insights for therapeutic strategies such as probiotics. Here, aiming to identify novel probiotics from the human gut microbes to combat T2DM, a total of 203 human intestinal bacterial strains were screened in vitro to evaluate their enzymatic inhibition of α-glucosidase and α-amylase, in which Lacticaseibacillus paracasei Glu-07 (= QH-142 = CGMCC 23796) exhibited the highest inhibitory efficacy (81.05% and 72.56% for α-glucosidase and α-amylase, respectively). In HepG2 cells, L. paracasei Glu-07 elevated glucose uptake and consumption by 22.4% and 85.8%. Oral administration of strain Glu-07 in diabetic mice significantly improved hyperglycemia, hyperlipidemia, and restored the structure and composition of gut microbiota toward a healthier profile. Mechanistically, strain Glu-07 activated the AMPK-AKT1-GSK3β/FOXO1 signaling pathways by modulating the expression of key genes, enhanced glycolysis and fatty acid ꞵ-oxidation, and inhibited gluconeogenesis and lipogenesis. It also stimulated GLP-1 secretion, potentially via triggering the GPR41/43-GLP-1 axis, and increased the abundance of beneficial gut microbes, such as Akkermansia muciniphila (~ threefold). The dual regulations synergistically support strain Glu-07 as a promising probiotic candidate for T2DM management.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Wenyi Xu, Yinghua Guan, Liping Tian, Yinghui Zhang, Yanhong Liu, and Bowen Zhao are employees of Beijing QuantiHealth Technology Co., Ltd. Yu Fu, Yanfei Hu, and Chongming Wu declare no competing interest. All data were objectively collected and analyzed, and study design, interpretation, and conclusions were independently validated to minimize potential bias.
Figures


Similar articles
-
Multi-strain probiotics attenuate carbohydrate-lipid metabolic dysregulation in type 2 diabetic rats via gut-liver axis modulation.mSystems. 2025 Jul 22;10(7):e0036925. doi: 10.1128/msystems.00369-25. Epub 2025 Jun 10. mSystems. 2025. PMID: 40492727 Free PMC article.
-
Oral delivery of GLP-1 peptide using recombinant Lactobacillus gasseri for the treatment of type 2 diabetes mellitus.Microbiol Spectr. 2025 Aug 5;13(8):e0282824. doi: 10.1128/spectrum.02828-24. Epub 2025 Jun 18. Microbiol Spectr. 2025. PMID: 40530879 Free PMC article.
-
Weizmannia coagulans SA9: A Novel Strategy to Alleviate Type 2 Diabetes.Nutrients. 2025 Jun 23;17(13):2081. doi: 10.3390/nu17132081. Nutrients. 2025. PMID: 40647186 Free PMC article.
-
Gut microbiome-based interventions for the management of obesity in children and adolescents aged up to 19 years.Cochrane Database Syst Rev. 2025 Jul 10;7(7):CD015875. doi: 10.1002/14651858.CD015875. Cochrane Database Syst Rev. 2025. PMID: 40637175 Review.
-
Synbiotics, prebiotics and probiotics for people with chronic kidney disease.Cochrane Database Syst Rev. 2023 Oct 23;10(10):CD013631. doi: 10.1002/14651858.CD013631.pub2. Cochrane Database Syst Rev. 2023. PMID: 37870148 Free PMC article.
References
-
- Khursheed R, Singh SK, Wadhwa S, Kapoor B, Gulati M, Kumar R, Ramanunny AK, Awasthi A, Dua K (2019) Treatment strategies against diabetes: success so far and challenges ahead. Eur J Pharmacol 862:172625. 10.1016/j.ejphar.2019.172625 - PubMed
-
- Fassatoui M, Saffarian A, Mulet C, Jamoussi H, Gamoudi A, Ben Halima Y, Hechmi M, Abdelhak S, Abid A, Sansonetti PJ et al (2023) Gut microbiota profile and the influence of nutritional status on bacterial distribution in diabetic and healthy Tunisian subjects. Biosci Rep 43:BSR20220803. 10.1042/BSR20220803 - PMC - PubMed
-
- Letchumanan G, Abdullah N, Marlini M, Baharom N, Lawley B, Omar MR, Mohideen FBS, Addnan FH, Nur Fariha MM, Ismail Z (2022) Gut microbiota composition in prediabetes and newly diagnosed type 2 diabetes: a systematic review of observational studies. Front Cell Infect Microbiol 12:943427. 10.3389/fcimb.2022.943427 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

