A comparison of electrophysiological microrecording versus automatic MR-based segmentation to determine subthalamic nucleus boundaries
- PMID: 40694157
- PMCID: PMC12283794
- DOI: 10.1007/s00701-025-06619-z
A comparison of electrophysiological microrecording versus automatic MR-based segmentation to determine subthalamic nucleus boundaries
Abstract
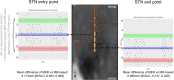
Purpose: Accurate placement of electrodes within the subthalamic nucleus is critical for deep brain stimulation (STN-DBS) in Parkinson's disease (PD). Our objective was to compare microelectrode recording (MER) and an automatic MR-based segmentation tool (BrainLab ElementsTM) for STN targeting and the determination of STN boundaries.
Methods: Seventy-eight PD patients were included. Electrode placement within the STN and STN entry and exit points were determined by both methods and compared for concordance.
Results: Of 344 trajectories, 269 were inside the STN, with good concordance of both techniques (Fleiss' kappa 0.721, [95%CI 0.623, 0.819]). Concordance of MER and MR-based for the selection of the optimal trajectory was good (Fleiss' kappa 0.693, [95%CI 0.578, 0.808]), with less than 2.75mm difference between MER and MR-based for the STN entry (upper limit of agreement 2.752 [95%CI 2.365 to 3.138] mm; lower limit of agreement -2.406 [95%CI -2.793 to -2.020] mm) and exit points (upper limit of agreement 2.750 [95%CI 2.351 to 3.149] mm; lower limit of agreement -2.577mm [95%CI -2.976 to -2.178]).
Conclusion: We demonstrated that MER and MR-based segmentation have a good concordance to determine STN boundaries during DBS surgery.
Keywords: Deep brain stimulation; Electrophysiology; Ideal trajectory; MRI-based segmentation; Parkinson’s disease.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Study approval statement: This study protocol was reviewed and approved by the local ethics committee of the Hospices Civils de Lyon (Comité Scientifique et Éthique des Hospices Civils de Lyon), IRB (Internal Review Board) 00013204, AGORA number 23-5439. Human ethics and consent to participate declaration: Informed consent was obtained from all the participants/legal guardian of participants prior to the study, to use their data in a completely anonymized form. Written informed consent was not deemed necessary because the study was conducted using regularly collected data. This study protocol and consent procedure was reviewed and approved by the local ethics committee of the Hospices Civils de Lyon (Comité Scientifique et Éthique des Hospices Civils de Lyon, Lyon, France), approval numbers IRB (Internal Review Board) 00013204, AGORA number 23-5439 (conformité MR004 n°23-5439), decision date 20/05/2024, and non-opposition was collected from patients. The study was in line with local and national guidelines. Our study has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Competing interests: C. de Laurentis declares that she has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. S. Thobois declares honoraria from Boston, Medtronic, Abbvie, Merz, NHC, MDS for consulting, board, or conferences; meeting and travel grant from the MDS and Abbvie. T. Danaila declares honoraria from Abbvie and Orkyn. C. Laurencin declares travel grants from Abbvie, and honoraria from Orphalan. G. Polo declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. S. Prange declares honoraria from Abbvie for consulting. E. Simon declares honoraria from Boston & BrainLab for conferences, meeting and travel grant from Boston.
Figures
Similar articles
-
Modulation of Cerebellar Oscillations with Subthalamic Stimulation in Patients with Parkinson's Disease.J Parkinsons Dis. 2024;14(7):1417-1426. doi: 10.3233/JPD-240065. J Parkinsons Dis. 2024. PMID: 39331106 Free PMC article.
-
Divide and Conquer: Automatic Detection of the Thalamus to Empower DBS Physiological Navigation to the Subthalamic Region.IEEE Trans Neural Syst Rehabil Eng. 2025;33:2672-2683. doi: 10.1109/TNSRE.2025.3582777. IEEE Trans Neural Syst Rehabil Eng. 2025. PMID: 40553676
-
Mild cognitive impairment is not predictive of dementia up to 15 years after subthalamic deep brain stimulation in Parkinson's disease.J Parkinsons Dis. 2025 Jun;15(4):879-891. doi: 10.1177/1877718X251334049. Epub 2025 May 20. J Parkinsons Dis. 2025. PMID: 40390641
-
Subthalamic nucleus and globus pallidus internus stimulation for the treatment of Parkinson's disease: A systematic review.J Int Med Res. 2017 Oct;45(5):1602-1612. doi: 10.1177/0300060517708102. Epub 2017 Jul 12. J Int Med Res. 2017. PMID: 28701061 Free PMC article.
-
Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes.Mov Disord. 2006 Jun;21 Suppl 14:S290-304. doi: 10.1002/mds.20962. Mov Disord. 2006. PMID: 16892449
References
-
- Benazzouz A, Breit S, Koudsie A, Pollak P, Krack P, Benabid A-L (2002) Intraoperative microrecordings of the subthalamic nucleus in Parkinson’s disease. Mov Disord 17:S145–S149. 10.1002/mds.10156 - PubMed
-
- Budnick HC, Schneider D, Zauber SE, Witt TC, Gupta K (2024) Susceptibility-Weighted MRI Approximates Intraoperative Microelectrode Recording During Deep Brain Stimulation of the Subthalamic Nucleus for Parkinson’s Disease. World Neurosurg 181:e346–e355. 10.1016/j.wneu.2023.10.053 - PubMed
-
- Chan H-L, Lin M-A, Lee S-T, Tsai Y-T, Chao P-K, Wu T (2010) Complex analysis of neuronal spike trains of deep brain nuclei in patients with Parkinson’s disease. Brain Res Bull 81:534–542. 10.1016/j.brainresbull.2010.01.001 - PubMed
-
- Chircop C, Dingli N, Aquilina A, Zrinzo L, Aquilina J (2018) MRI-verified “asleep” deep brain stimulation in Malta through cross border collaboration: clinical outcome of the first five years. Br J Neurosurg 32:365–371. 10.1080/02688697.2018.1478061 - PubMed
-
- Coenen VA, Reisert M (2021) Chapter Three - DTI for brain targeting: Diffusion weighted imaging fiber tractography—Assisted deep brain stimulation. In: Moro E, Polosan M, Hamani C (eds) International Review of Neurobiology. Academic Press, pp 47–67 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

