Combination of PSMA targeting alpha-emitting radioligand [212Pb]Pb-AB001 with BET bromodomain inhibitors in in vitro prostate cancer models
- PMID: 40694184
- PMCID: PMC12283873
- DOI: 10.1007/s12032-025-02925-9
Combination of PSMA targeting alpha-emitting radioligand [212Pb]Pb-AB001 with BET bromodomain inhibitors in in vitro prostate cancer models
Abstract
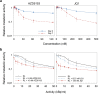
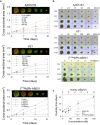
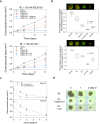
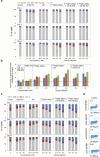
The alpha-emitting radioligand [212Pb]Pb-AB001, targeting prostate-specific membrane antigen (PSMA), is a promising therapy approach for prostate cancer. Bromodomain and extra-terminal (BET) protein inhibitors, such as AZD5153 and JQ1, disrupt oncogenic transcriptional programs by altering chromatin structure. This study evaluated whether BET inhibition enhances the efficacy of radioligand therapy. Cytotoxic effects of [212Pb]Pb-AB001 alone and in combination with BET inhibitors were assessed in 2D monolayers and a 3D spheroid model of PSMA-positive C4-2 prostate cancer cells. Cell viability, cell cycle alterations, and DNA damage were assessed using viability assays and flow cytometry. Spheroid growth and viability were assessed by fluorescence microscopy. AZD5153 was more potent than JQ1 in reducing cell proliferation. [212Pb]Pb-AB001 induced activity- and time-dependent cytotoxicity with a delayed apoptotic response. BET inhibitors induced G1 arrest, while [212Pb]Pb-AB001 caused G2 arrest. Combination treatment reduced cell viability in an additive manner but did not further affect cell cycle distribution or increase apoptosis compared with [212Pb]Pb-AB001 alone. γH2AX staining in 2D models showed an activity- and time-dependent increase in DNA damage at 1, 3 and 6 days post-treatment with [212Pb]Pb-AB001. BET inhibitors alone induced minimal γH2AX, and combination treatments did not enhance DNA damage beyond [212Pb]Pb-AB001 alone. In 3D spheroids, combination treatment led to synergistic growth suppression. In conclusion, these findings indicate that therapeutic inhibition of BET bromodomain in combination with the alpha-emitting radioligand [212Pb]Pb-AB001 could significantly enhance tumour control and should be further evaluated in metastatic prostate cancer models.
Keywords: Bromodomain and extra-terminal proteins; Cell cycle; Combination treatment; Targeted alpha therapy; Targeted radionuclide therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests. Ethical approval: Not applicable. Consent to participate: Not Applicable. Consent for publication: All authors consent to the publication of this.
Figures
Similar articles
-
Targeting Myc through BET-PROTAC elicits potent anti-lymphoma activity in diffuse large B cell lymphoma.Invest New Drugs. 2025 Jun;43(3):621-633. doi: 10.1007/s10637-025-01535-6. Epub 2025 May 1. Invest New Drugs. 2025. PMID: 40307411
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
In Vitro and In Vivo Study of Novel PSMA-Targeted Radioligands: Enhancing Tumor Uptake and Therapeutic Efficacy through Zwitterionization and Albumin-Binding Strategies.Mol Pharm. 2025 Jul 7;22(7):3961-3975. doi: 10.1021/acs.molpharmaceut.5c00214. Epub 2025 Jun 10. Mol Pharm. 2025. PMID: 40494649
-
A third generation PSMA-targeted agent [211At]YF2: Synthesis and in vivo evaluation.Nucl Med Biol. 2024 Jul-Aug;134-135:108916. doi: 10.1016/j.nucmedbio.2024.108916. Epub 2024 May 1. Nucl Med Biol. 2024. PMID: 38703587 Free PMC article.
-
A Comprehensive Systematic Review and Meta-analysis of the Role of Prostate-specific Membrane Antigen Positron Emission Tomography for Prostate Cancer Diagnosis and Primary Staging before Definitive Treatment.Eur Urol. 2025 Jun;87(6):654-671. doi: 10.1016/j.eururo.2025.03.003. Epub 2025 Mar 27. Eur Urol. 2025. PMID: 40155242
References
-
- Filho AM, et al. The GLOBOCAN 2022 cancer estimates: data sources, methods, and a snapshot of the cancer burden worldwide. Int J Cancer. 2024;156:1336. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

