Efficacy and safety of doxycycline for severe Mycoplasma pneumoniae pneumonia in pediatric patients
- PMID: 40694196
- PMCID: PMC12283874
- DOI: 10.1007/s10238-025-01793-x
Efficacy and safety of doxycycline for severe Mycoplasma pneumoniae pneumonia in pediatric patients
Abstract
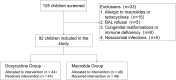
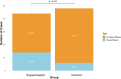
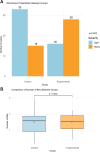
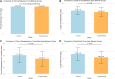
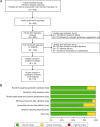
This study evaluated the efficacy and safety of doxycycline in treating Severe Mycoplasma pneumoniae Pneumonia (SMPP) in children under eight years old through clinical analysis and meta-analysis. A total of 92 pediatric SMPP cases were divided into a doxycycline treatment group (44 cases) and a macrolides control group (48 cases). Compared to the control group, the doxycycline group exhibited significantly shorter cough relief time (5.4 ± 1.2 vs. 7.2 ± 1.6 days, p < 0.05) and pulmonary rale resolution time (6.2 ± 1.3 vs. 8.0 ± 1.7 days, p < 0.05). The overall treatment efficacy rate was higher in the doxycycline group (88.6% vs. 75.0%, p < 0.05). No significant differences were found in fever resolution time or hospitalization duration (p > 0.05). Safety analysis revealed comparable adverse event rates between groups (18.2% vs. 16.7%, p > 0.05), primarily mild rash and gastrointestinal discomfort, with no tooth discoloration observed. The meta-analysis confirmed the advantages of doxycycline, demonstrating superior treatment efficacy (RR: 0.68, 95% CI: 0.58-0.79), shorter fever resolution (MD: - 1.5 days, 95% CI: - 2.3 to - 0.7), and faster cough and pulmonary rale resolution. Adverse events were similar across groups. These findings highlight doxycycline's clinical efficacy and safety in SMPP treatment, providing strong evidence for its application in pediatric practice.
Keywords: Antibiotic therapy in children; Doxycycline; Efficacy and safety; Meta-analysis; Pediatric respiratory infection; Severe Mycoplasma pneumoniae pneumonia.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests. Ethical approval: This study was approved by the Clinical Ethics Committee of The First Affiliated Hospital of Bengbu Medical University (No. [2024] 212).
Figures


Similar articles
-
Exploration of the Therapeutic Efficacy of Azithromycin Sequential Therapy in Children With Mycoplasma Pneumonia.Br J Hosp Med (Lond). 2025 Jun 25;86(6):1-18. doi: 10.12968/hmed.2025.0005. Epub 2025 Jun 13. Br J Hosp Med (Lond). 2025. PMID: 40554432
-
Respiratory microbiome and metabolome features associate disease severity and the need for doxycycline treatment in children with macrolide-resistant Mycoplasma pneumoniae-mediated pneumonia.Front Cell Infect Microbiol. 2025 Jul 28;15:1537182. doi: 10.3389/fcimb.2025.1537182. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40792100 Free PMC article.
-
Clinical features associated with macrolides resistance of Mycoplasma pneumoniae pneumonia in children: a single-center analysis.BMC Infect Dis. 2025 Jul 1;25(1):854. doi: 10.1186/s12879-025-11234-5. BMC Infect Dis. 2025. PMID: 40597734 Free PMC article.
-
Antibiotics for treating scrub typhus.Cochrane Database Syst Rev. 2018 Sep 24;9(9):CD002150. doi: 10.1002/14651858.CD002150.pub2. Cochrane Database Syst Rev. 2018. PMID: 30246875 Free PMC article.
-
Prophylactic antibiotics for adults with chronic obstructive pulmonary disease: a network meta-analysis.Cochrane Database Syst Rev. 2021 Jan 15;1(1):CD013198. doi: 10.1002/14651858.CD013198.pub2. Cochrane Database Syst Rev. 2021. PMID: 33448349 Free PMC article.
References
-
- Krafft C, Christy C. Mycoplasma pneumonia in children and adolescents. Pediatr Rev. 2020;41:12–9. 10.1542/pir.2018-0016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2023byzd076/Bengbu Medical University Natural Science Program
- 2023byzd076/Bengbu Medical University Natural Science Program
- 2023byzd076/Bengbu Medical University Natural Science Program
- 2023byzd076/Bengbu Medical University Natural Science Program
- 2023byzd076/Bengbu Medical University Natural Science Program
LinkOut - more resources
Full Text Sources
Medical

