Real-world treatment patterns for patients with non-infectious uveitis in Japan: a descriptive study using a large-scale claims database (J-CAT study)
- PMID: 40694217
- PMCID: PMC12283504
- DOI: 10.1186/s12348-025-00514-5
Real-world treatment patterns for patients with non-infectious uveitis in Japan: a descriptive study using a large-scale claims database (J-CAT study)
Abstract
Purpose: Non-infectious uveitis (NIU) can arise from various inflammatory disorders and can cause vision loss. Patients with mild NIU are typically treated with corticosteroid eye drops to reduce intraocular inflammation; however, other local/systemic treatments (corticosteroids, immunosuppressants, biologics) may be required for moderate-to-severe NIU, which may cause ocular complications. Here, we investigated real-world treatment patterns for NIU in Japan.
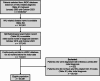
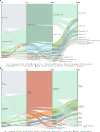
Methods: Patients were selected from a large, Japanese insurance claims database using International Classification of Diseases, Tenth Revision codes; diagnosis of NIU was confirmed via ophthalmological examination (October 2016-October 2023). Sankey diagrams were used to describe treatment transitions. Post-treatment ocular complications, potentially related surgeries, and fundus findings associated with uveitis were determined.
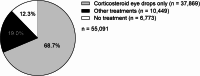
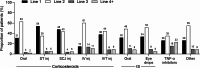
Results: The majority of patients (68.7%; 37,869/55,091) were treated with corticosteroid eye drops only for mild NIU; 19.0% (10,449/55,091) were given other treatments for moderate-to-severe NIU, mostly oral corticosteroids (7,473/10,449) and posterior sub-Tenon's corticosteroid injections (1,636/10,449). In patients treated with corticosteroids orally or via sub-Tenon's injections, common transitions were to corticosteroid eye drops or censor (end of treatment/dataset or insurance withdrawal). A higher incidence of treatment-related ocular complications and potentially related surgeries (including glaucoma) was observed during the first year of NIU treatment compared with subsequent years (for moderate-to-severe NIU, estimated incidence of prescription of glaucoma drugs was 106 per 1,000 person-years [at 1 year], 73 per 1,000 person-years [at 2 years], and 52 per 1,000 person-years [at 5 years]).
Conclusion: Our comprehensive analysis of a large claims database included all prescribed medications and medical procedures (including local injections) for NIU treatment in Japan up to October 2023. Although corticosteroids are a mainstay of NIU treatment in Japan, we found that a number of treatments for moderate-to-severe NIU, other than corticosteroid eye drops, are frequently used in combination with or when switching from corticosteroid eye drops. These findings are of importance when assessing the treatment landscape and may help identify unmet clinical needs in patients with NIU.
Keywords: Corticosteroid; Glaucoma; Japan; Non-infectious uveitis; Treatment patterns.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: As the JMDC database comprises de-identified data, informed consent is not required. The study was exempt from review by the Institutional Review Board of the Kobe University Graduate School of Medicine Consent for publication: Not applicable. Competing interests: S.Kusuhara: payment for lectures or supervision from AbbVie GK., AMO Japan K.K., Bayer Yakuhin, Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Company, Ltd., Eisai Co., Ltd., Kowa Company, Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Nippon Kayaku, Novartis Pharma K.K., Santen Pharmaceutical Co., Ltd., Senju Pharmaceutical Co., Ltd., and Viatris Inc.; grants from AMO Japan K.K., Bayer Yakuhin, Ltd., and Chugai Pharmaceutical Co., Ltd.; receipt of drugs from Clinigen K.K.; patent royalties from Charmant, Inc.; K.Sonoda: financial support from AbbVie GK., Alcon Japan Ltd., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., HOYA Corporation, Mitsubishi Tanabe Pharma Corporation, Otsuka Pharmaceutical Corporation, Santen Pharmaceutical Co., Ltd., Senju Pharmaceutical Co., Ltd., and Wakamoto Pharmaceutical Corporation; review panel member for Chugai Pharmaceutical Co., Ltd., Japan Tobacco Inc., Riverfield Corporation, Sand Corporation, Santen Pharmaceutical Co., Ltd., and Senju Pharmaceutical Co., Ltd.; T.K.: payment for lectures or supervision from AbbVie GK., Bayer Yakuhin, Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Company, Ltd., Eisai Co., Ltd., Kowa Company, Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Kayaku, Novartis Japan, Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Santen Pharmaceutical Co., Ltd., and Senju Pharmaceutical Co., Ltd.; grants from AbbVie GK., Alcon Japan Ltd., Bayer Yakuhin, Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Company, Ltd., HOYA Corporation, Santen Pharmaceutical Co., Ltd., and Senju Pharmaceutical Co., Ltd.; receipt of drugs from Kowa Company, Ltd.; T.F., S.Katayama, M.M., T.N., M.N., T.O., Y.T., T.Y.: employees of Chugai Pharmaceutical Co., Ltd.; K.Sasaki, K.R.: employees of Datack, Inc., a paid vendor of Chugai Pharmaceutical Co., Ltd.; indirectly received consulting fees from Chugai Pharmaceutical Co., Ltd. through Datack, Inc.; K.Sato: employee of Third Place, LLC; received consulting fees from Datack, Inc.; H.G.: payment for lectures or supervision from AbbVie GK., Chugai Pharmaceutical Co., Ltd., Kowa Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Santen Pharmaceutical Co., Ltd., Senju Pharmaceutical Co., Ltd.; financial support from AbbVie GK., Alcon Japan Ltd., Chugai Pharmaceutical Co., Ltd., HOYA Corporation, Santen Pharmaceutical Co., Ltd., and Senju Pharmaceutical Co., Ltd.
Figures




Similar articles
-
Corticosteroid implants for chronic non-infectious uveitis.Cochrane Database Syst Rev. 2023 Jan 16;1(1):CD010469. doi: 10.1002/14651858.CD010469.pub3. Cochrane Database Syst Rev. 2023. Update in: Cochrane Database Syst Rev. 2023 Aug 29;8:CD010469. doi: 10.1002/14651858.CD010469.pub4. PMID: 36645716 Free PMC article. Updated.
-
Non-steroidal anti-inflammatory drugs versus corticosteroids for controlling inflammation after uncomplicated cataract surgery.Cochrane Database Syst Rev. 2017 Jul 3;7(7):CD010516. doi: 10.1002/14651858.CD010516.pub2. Cochrane Database Syst Rev. 2017. PMID: 28670710 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Fornix-based versus limbal-based conjunctival trabeculectomy flaps for glaucoma.Cochrane Database Syst Rev. 2015 Nov 25;11(11):CD009380. doi: 10.1002/14651858.CD009380.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2021 Aug 26;8:CD009380. doi: 10.1002/14651858.CD009380.pub3. PMID: 26599668 Free PMC article. Updated.
-
Corticosteroid implants for chronic non-infectious uveitis.Cochrane Database Syst Rev. 2016 Feb 12;2(2):CD010469. doi: 10.1002/14651858.CD010469.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2023 Jan 16;1:CD010469. doi: 10.1002/14651858.CD010469.pub3. PMID: 26866343 Free PMC article. Updated.
References
-
- Sonoda KH, Hasegawa E, Namba K, Okada AA, Ohguro N, Goto H (2021) Epidemiology of uveitis in japan: a 2016 retrospective nationwide survey. Jpn J Ophthalmol 65(2):184–190 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

