Ixekizumab Improves Signs, Symptoms, and Quality of Life in Patients with Axial Spondyloarthritis Irrespective of Symptom Duration
- PMID: 40694276
- PMCID: PMC12394305
- DOI: 10.1007/s12325-025-03305-5
Ixekizumab Improves Signs, Symptoms, and Quality of Life in Patients with Axial Spondyloarthritis Irrespective of Symptom Duration
Abstract
Introduction: The objective of this study was to assess treatment response to ixekizumab, an interleukin-17A antagonist, by shorter versus longer symptom duration (< 5 years vs. ≥ 5 years) in patients with radiographic axial spondyloarthritis (r-axSpA) and non-radiographic axial spondyloarthritis (nr-axSpA) up to 52 weeks.
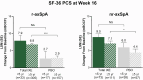
Methods: This post hoc analysis used data from three randomized, placebo-controlled trials including patients with r-axSpA from COAST-V [biologic disease-modifying anti-rheumatic drug (bDMARD)-naïve] and COAST-W (tumor necrosis factor inhibitor-experienced) and patients with nr-axSpA from COAST-X (bDMARD-naïve). Patients received ixekizumab (80 mg every 2 or 4 weeks) or placebo through Week 16 and ixekizumab to Week 52. Assessments included the Assessment in SpondyloArthritis international Society 40% improvement (ASAS40) and Axial Spondyloarthritis Disease Activity Score (ASDAS) low disease activity [LDA (< 2.1)] and Bath Ankylosing Spondylitis Disease Activity Index 50% improvement (BASDAI50) response rates through Week 52 and change from baseline in 36-Item Short Form Health Survey (SF-36) Physical Component Summary (PCS) at Week 16.
Results: Fewer patients treated with ixekizumab (pooled dosing) had shorter versus longer symptom duration [n = 33 vs. n = 306 (r-axSpA); n = 73 vs. n = 111 (nr-axSpA)]. Ixekizumab-treated patients with shorter versus longer symptom duration had numerically higher response rates at Week 16/Week 52 for ASAS40 [51.5/60.6 vs. 36.9/40.5 (r-axSpA); 42.5/54.8 vs. 36.0/41.4 (nr-axSpA)], ASDAS LDA [39.4/48.5 vs. 27.5/35.6 (r-axSpA); 32.9/49.3 vs. 27.9/36.9 (nr-axSpA)], and BASDAI50 [42.4/54.5 vs. 31.4/36.6 (r-axSpA); 38.4/49.3 vs. 27.9/34.2 (nr-axSpA)]. However, relative risk ratios at Week 16 did not significantly favor the shorter duration subgroup. Findings were comparable for SF-36 PCS at Week 16. The present findings should be interpreted in the context of small numbers of patients in some shorter duration subgroups.
Conclusion: Ixekizumab was shown to be efficacious in both patients with shorter or longer symptom duration and in r-axSpA or nr-axSpA.
Trial registration: ClinicalTrials.gov Identifiers, NCT02696785; NCT02696798; NCT02757352.
Keywords: Axial spondyloarthritis; Interleukin-17; Ixekizumab; Non-radiographic axial spondyloarthritis.
Plain language summary
Axial spondyloarthritis is a type of arthritis that primarily affects the sacroiliac joints and spine, causing stiffness and pain. In severe cases, the joints and bones may eventually fuse together. Patients with axial spondyloarthritis may or may not have joint damage that is visible on X-rays (radiographic vs. non-radiographic disease). In previous studies, we showed that ixekizumab is an effective treatment for both radiographic and non-radiographic axial spondyloarthritis. In this study, we examined whether patients respond differently based on shorter (fewer than 5 years) versus longer (5 years or more) symptom duration with radiographic and non-radiographic axial spondyloarthritis. We analyzed data collected previously from three clinical trials of patients with radiographic or non-radiographic axial spondyloarthritis who were treated with ixekizumab for up to 52 weeks. The patients who had experienced disease symptoms for fewer than 5 years showed similar improvements with ixekizumab treatment as the patients who had experienced symptoms for 5 years or more. Our results suggest that ixekizumab is an effective treatment for patients with axial spondyloarthritis, regardless of whether they are in the earlier or later stages of the disease course.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Victoria Navarro-Compán has served as a speaker, consultant, and/or instructor for AbbVie, Alfasigma, Eli Lilly and Company, Fresenius Kabi, Galapagos, Janssen, Novartis, Pfizer, and UCB Pharma, and has received grant and/or research support from ASAS and Novartis. John D. Reveille has served as a consultant for Eli Lilly and Company and has received grant and/or research support from Eli Lilly and Company. Proton Rahman has served as a speaker and instructor for Abbott, AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Janssen, Novartis, Pfizer, and UCB Pharma, and has received grant and/or research support from Janssen and Novartis. José A. Maldonado-Cocco has served as a speaker and consultant for and has received grant and/or research support from AbbVie, Bristol Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Sanofi-Aventis, Schering-Plough, UCB Pharma, and Wyeth. Marina Magrey has served as a speaker and/or consultant for AbbVie, Bristol Myers Squibb, Eli Lilly and Company, Janssen, Novartis, Pfizer, and UCB Pharma. Rebecca Bolce is a former and Tommaso Panni, and Andris Kronbergs are current employees and shareholders of Eli Lilly and Company. Martin Rudwaleit has served as a speaker, consultant, and/or instructor for AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Chugai Pharmaceutical, Eli Lilly and Company, Janssen, Novartis, Pfizer, and UCB Pharma. Ethical Approval: The trials described herein were conducted in accordance with the ethical principles of the Declaration of Helsinki and were approved by the ethics review boards at each participating study site. The master ethics committee was Schulman Associates IRB, Cincinnati, OH, USA (IRB reference numbers: COAST-V, 201506061; COAST-W, 201506079; COAST-X, 201601302). All patients provided written informed consent prior to participating in the trials.
Figures

References
-
- Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390(10089):73–84. - PubMed
-
- Baraliakos X, Koenig AS, Jones H, Szumski A, Collier D, Bananis E. Predictors of clinical remission under anti-tumor necrosis factor treatment in patients with ankylosing spondylitis: pooled analysis from large randomized clinical trials. J Rheumatol. 2015;42(8):1418–26. - PubMed
-
- Rudwaleit M, Claudepierre P, Wordsworth P, et al. Effectiveness, safety, and predictors of good clinical response in 1250 patients treated with adalimumab for active ankylosing spondylitis. J Rheumatol. 2009;36(4):801–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

