G Proteins-Associated Dose-Dependent Effects of Oxytocin on Oxytocin Neuronal Activity and Astrocytic Plasticity of the Supraoptic Nucleus
- PMID: 40694283
- PMCID: PMC12559076
- DOI: 10.1007/s12035-025-05193-w
G Proteins-Associated Dose-Dependent Effects of Oxytocin on Oxytocin Neuronal Activity and Astrocytic Plasticity of the Supraoptic Nucleus
Abstract
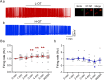
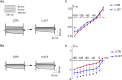
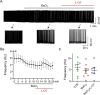
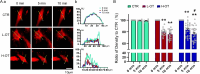
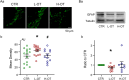
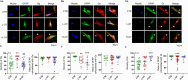
Dose-dependent neuromodulation has been well established; however, the molecular mechanisms underlying astrocytic involvement in this process remain largely unexplored. Using the autoregulation of supraoptic oxytocin (OT) neurons (OTNs) as a model, we investigated the role of distinct astrocytic G proteins and their targets in the dose-dependent effects of OT on OTN activity. The results showed that OT in a low concentration (10 pmol/L, L-OT) excited OTN activity, whereas a high concentration (1 nmol/L, H-OT) inhibited it in brain slices. These effects were abolished upon disruption of astrocytic plasticity using L-aminoadipic acid, a gliotoxin. In primary astrocyte cultures, L-OT slightly reduced the current through astrocyte-specific inwardly rectifying K⁺ channel 4.1 (Kir4.1) while H-OT strongly enhanced it. Selectively blocking Kir4.1 with BaCl₂ (100 µmol/L) did not affect the basal activity but blocked the excitatory effect of L-OT in brain slices. In cultured astrocytes, L-OT mobilized Gαq subunit expression, increased glial fibrillary acidic protein (GFAP) filaments, and quickly expanded astrocytic volume, predominantly visible at the somata. Conversely, H-OT released Gαi subunits and induced progressive volume expansion. Pretreatment of brain slices with U73122 (a Gq inhibitor) or SQ22536 (a Gs inhibitor) suppressed L-OT-induced excitation. Conversely, activation of adenylyl cyclase with forskolin reversed the inhibitory effect of H-OT, and inhibition of Gi with pertussis toxin blocked H-OT-induced inhibition. These findings imply that the dose-dependent effects of OT on OTN activity are mediated, at least partially, by different receptor-coupled G proteins and their subsequent modulation of astrocytic Kir4.1 currents, GFAP expression, and volume dynamics. This mechanism underlying the autoregulation of OTN activity provides an important reference for understanding the concentration-dependent neuromodulation.
Keywords: Excitability; G protein; Glia; Hypothalamus; Neurons; Oxytocin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval: This study was performed in accordance with the Guideline of National Institutes of Health and approved by the Institutional Animal Care and Use Committees of Harbin Medical University (HMU2023-03–05). Competing interests: The authors declare no competing interests.
Figures


References
-
- Higuchi T, Tadokoro Y, Honda K, Negoro H (1986) Detailed analysis of blood oxytocin levels during suckling and parturition in the rat. J Endocrinol 110(2):251–256 - PubMed
-
- Augustine RA, Seymour AJ, Campbell RE, Grattan DR, Brown CH (2018) Integrative neuro-humoral regulation of oxytocin neuron activity in pregnancy and lactation. J Neuroendocrinol. 10.1111/jne.12569 - PubMed
-
- Leng G, MacGregor DJ (2018) Models in neuroendocrinology. Math Biosci 305:29–41. 10.1016/j.mbs.2018.07.008 - PubMed
MeSH terms
Substances
Grants and funding
- YQJH2023031/Excellent Young Teachers Basic Research Support Program of Heilongjiang Provincial
- XYL, 2023/the Fundamental Research Funds for the Provincial Universities
- JJ2023LH1248/Core Teacher Program of Harbin Medical University
- G2021011014L/the Fund of the Ministry of Science and Technology of China
LinkOut - more resources
Full Text Sources
Miscellaneous

