TMEM63A, associated with hypomyelinating leukodystrophies, is an evolutionarily conserved regulator of myelination
- PMID: 40694323
- PMCID: PMC12318207
- DOI: 10.1073/pnas.2507354122
TMEM63A, associated with hypomyelinating leukodystrophies, is an evolutionarily conserved regulator of myelination
Abstract
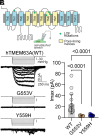
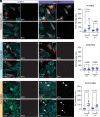
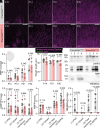
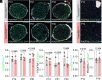
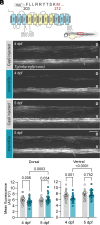
Infantile hypomyelinating leukodystrophy 19 (HLD19) is a rare genetic disorder where patients exhibit reduced myelin in central nervous system (CNS) white matter tracts and present with varied neurological symptoms. The causative gene TMEM63A encodes a mechanosensitive ion channel whose role in myelination is largely unexplored. Our study shows that TMEM63A is a major regulator of oligodendrocyte (OL)-dependent myelination in the CNS. In mouse and zebrafish, Tmem63a inactivation led to early deficits in myelination, recapitulating the HLD19 phenotype. OL-specific conditional mouse knockouts of Tmem63a exhibited transient reductions in myelin, indicating that TMEM63A regulates myelination cell-autonomously. We show that TMEM63A is present at the plasma membrane and on lysosomes and modulates myelin production in the presence of mechanical cues. Intriguingly, HLD19-associated TMEM63A variants from patients blocked trafficking to cell membrane. Together, our results reveal an ancient role for TMEM63A in fundamental aspects of myelination in vivo and highlight two exciting models for the development of treatments for devastating hypomyelinating leukodystrophies.
Keywords: TMEM63A; leukodystrophies; mechanosensitive ion channels; myelin; oligodendrocytes.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Tonduti D., et al. , Spinal cord involvement and paroxysmal events in "Infantile Onset Transient Hypomyelination" due to TMEM63A mutation. J. Hum. Genet. 66, 1035–1037 (2021). - PubMed
-
- Fukumura S., et al. , A novel de novo TMEM63A variant in a patient with severe hypomyelination and global developmental delay. Brain Dev. 44, 178–183 (2022). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

