Enhancing outcomes in mCRPC: the impact of androgen receptor inhibitor sequencing before 177Lu-PSMA-617 therapy
- PMID: 40694485
- PMCID: PMC12404291
- DOI: 10.1093/oncolo/oyaf213
Enhancing outcomes in mCRPC: the impact of androgen receptor inhibitor sequencing before 177Lu-PSMA-617 therapy
Abstract
Background: Prostate-specific membrane antigen (PSMA) is a key target in metastatic castration resistance prostate cancer (mCRPC). Enzalutamide, an androgen receptor pathway inhibitor (ARPi), increases PSMA expression, potentially enhancing 177Lu-PSMA-617 radioligand therapy. This study evaluates the impact of prior ARPi (enzalutamide vs abiraterone acetate [AA]) on PSMA expression, PFS, and OS.
Materials and methods: A retrospective analysis of 214 mCRPC patients treated with 177Lu-PSMA-617 across six Turkish centers (2015-2025) was conducted. Patients were grouped by prior ARPi therapy. PFS and OS were analyzed using Kaplan-Meier and Cox regression methods.
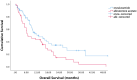
Results: Among 103 patients receiving ARPi before 177Lu-PSMA-617, 59 (57%) had enzalutamide and 44 (43%) AA. Median PFS was 7.6 months for enzalutamide versus 5.3 months for AA (P = .068). Median OS was significantly longer with enzalutamide (12.8 vs 6.9 months, P = .021). Patients with Eastern Cooperative Oncology Group Performance Scores (ECOG PS) 0-1 had significantly longer OS (27.6 vs 6.9 months for PS 2-3, P < .0001). Higher PSMA SUVmax (>20) correlated with longer OS (15.1 vs 7.8 months, P = .016). Among 86 patients with detectable PSMA SUVmax, 53 had SUVmax > 20; 66% had prior enzalutamide and 34% AA. Median OS was four months longer with enzalutamide (18.1 vs 13.9 months P = .120). Multivariate analysis identified ARPi type (HR: 2.24, P = .033) and ECOG PS (HR: 5.22, P < .0001) as independent OS predictors.
Conclusion: Enzalutamide prior to 177Lu-PSMA-617 significantly improves OS and enhances PSMA expression compared to AA. These findings highlight the importance of treatment sequencing in mCRPC and warrant further prospective studies.
Keywords: 177Lu-PSMA-617; ARPi; Abiraterone acetate; Enzalutamide; PSMA.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
Yüksel Ürün declared research funding (Institutional and personal) from Turkish Oncology Group. Yüksel Ürün has served on the advisory board for Abdi-İbrahim, Astellas, AstraZeneca, Bristol Myers-Squibb, Eczacıbası, Gilead, Janssen, Merck, Novartis, Pfizer, Roche. Yüksel Ürün received honoraria or has served as a consultant for Abdi-İbrahim, Astellas, Bristol Myers-Squibb, Eczacıbasi, Janssen, Merck, Novartis, Pfizer, Roche. All remaining authors have declared no conflicts of interest.
Figures
References
-
- Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528-539. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous