Immunogenicity and Safety of Heterologous Versus Homologous Prime-Boost Regimens With BBIBP-CorV and Ad26.COV2.S COVID-19 Vaccines: A Multicentric, Randomized, Observer-Blinded Non-inferiority Trial in Madagascar and Mozambique
- PMID: 40694514
- PMCID: PMC12282515
- DOI: 10.1093/cid/ciaf130
Immunogenicity and Safety of Heterologous Versus Homologous Prime-Boost Regimens With BBIBP-CorV and Ad26.COV2.S COVID-19 Vaccines: A Multicentric, Randomized, Observer-Blinded Non-inferiority Trial in Madagascar and Mozambique
Abstract
Background: Data on immunogenicity and safety of heterologous prime-boost (HePB) regimens using the BBIBP-CorV and Ad26.COV2.S have not yet been reported in sub-Saharan Africa.
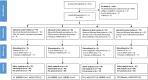
Methods: We conducted a randomized, observer-blinded, non-inferiority trial assessing the immunogenicity and safety of HePB regimens using BBIBP-CorV and Ad26.COV2.S, in adults aged 18-65 years. Participants enrolled, were stratified by baseline severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) serostatus, and randomized into four arms in a 1:1:1:1 ratio: A1 (BBIBP-CorV, Ad26.COV2.S), A2 (BBIBP-CorV, BBIBP-CorV), B1 (Ad26.COV2.S, BBIBP-CorV), and B2 (placebo, Ad26.COV2.S), administered at 28-day intervals. Fifteen participants in each arm were randomized separately in the immunology subset at a ratio of 1:1:1:1. Primary endpoints were the geometric mean titers (GMTs) of anti-SARS-CoV-2 neutralizing antibodies (nAbs) against SARS-CoV-2 Omicron variant BA.1 and safety at 4 weeks after second vaccination. The non-inferiority margin was 0.67 fold difference in geometric mean ratio (GMR) between the ratio of GMTs in the heterologous versus corresponding homologous arms.
Results: A total of 369 participants were randomized, and 367 of them received at least one dose of vaccine. Participants were between 18 and 65 years of age. Four weeks after second dose, GMT of nAbs in arms A1 and A2 was 802.7 (95% confidence interval [CI]: 635.3-1014.3) and 202.6 (95% CI: 150.8-272.1), respectively, with an adjusted GMR of 4.2 (2-sided 95% CI: 2.9-5.9). GMTs were 603.6 (95% CI: 446.1-816.7) and 725.7 (95% CI: 539.5-976.1) in arms B1 and B2, respectively, with an adjusted GMR of 0.8 (2-sided 95% CI: .5-1.2). Three serious adverse events were reported and none of them were related to the vaccination.
Conclusions: The noninferiority criterion was met only in arm A1 versus A2. HePB regimens were safe and well tolerated.
Clinical trials registration: NCT04998240.
Keywords: Ad26.COV2.S; BBIBP-CorV; SARS-CoV-2; heterologous; homologous.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Figures

Similar articles
-
Efficacy and safety of COVID-19 vaccines.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD015477. doi: 10.1002/14651858.CD015477. Cochrane Database Syst Rev. 2022. PMID: 36473651 Free PMC article.
-
Safety and immunogenicity of Ad26.COV2.S in adults: A randomised, double-blind, placebo-controlled Phase 2a dose-finding study.Vaccine. 2024 Jun 11;42(16):3536-3546. doi: 10.1016/j.vaccine.2024.04.059. Epub 2024 May 4. Vaccine. 2024. PMID: 38705804 Clinical Trial.
-
Heterologous Ad26.COV2.S booster after primary BBIBP-CorV vaccination against SARS-CoV-2 infection: 1-year follow-up of a phase 1/2 open-label trial.Vaccine. 2024 Jul 25;42(19):3999-4010. doi: 10.1016/j.vaccine.2024.05.010. Epub 2024 May 13. Vaccine. 2024. PMID: 38744598 Clinical Trial.
-
Hybrid Immunity in a Mozambican Cohort After 1 or 2 Doses of the BBIBP-CorV Vaccine.Clin Infect Dis. 2025 Jul 22;80(Supplement_1):S57-S65. doi: 10.1093/cid/ciaf095. Clin Infect Dis. 2025. PMID: 40694517 Free PMC article.
-
COVID-19 Vaccines.2025 Jul 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. 2025 Jul 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. PMID: 33355732 Free Books & Documents. Review.
References
-
- Josephson A, Kilic T, Michler JD. Socioeconomic impacts of COVID-19 in low-income countries. Nat Hum Behav 2021; 5:557–65. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

