Enhancing Clinical Trial Sites in Low- and Middle-Income Countries to Facilitate Product Development in Response to the COVID-19 Pandemic
- PMID: 40694516
- PMCID: PMC12282514
- DOI: 10.1093/cid/ciaf094
Enhancing Clinical Trial Sites in Low- and Middle-Income Countries to Facilitate Product Development in Response to the COVID-19 Pandemic
Abstract
Background: The swift development of coronavirus disease 2019 (COVID-19) vaccines marked a monumental effort in global coordination and collaboration; however, there remained major disparities in vaccine access and research capacity across countries. Unequal participation in vaccine development studies from low- and middle- income countries (LMICs) clearly signaled an urgent need to strengthen health research infrastructure in those regions.
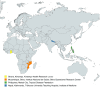
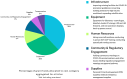
Methods: With funding from the Gates Foundation (GF), this site readiness initiative carried out rapid capacity enhancement activities to enable large-scale, Phase 3 pivotal clinical trial conduct in LMICs. The International Vaccine Institute (IVI) worked with site partners in four countries (Mozambique, Ghana, Nepal, and the Philippines) after conducting feasibility assessments for site selection. Site-specific gaps were identified, and capacity building activities focused on staff training, site infrastructure, and resource mobilization were carried out over roughly 7 months from October 2020 to May 2021.
Results: Despite pandemic-related challenges such as supply chain shortages, by the end of the capacity building efforts all sites were either contracted to or in discussions with trial sponsors to conduct severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine studies. This article provides an overview of the site selection process, critical components of site establishment, and final site readiness evaluations carried out amidst a global health emergency.
Conclusions: This experience illustrates the value of research capacity enhancement as essential to both pandemic preparedness and global health equity. The lessons learned are being carried into an ongoing initiative across West Africa, currently underway as the "Advancing Research Capabilities in West Africa (ARC-WA)."
Keywords: COVID-19; capacity enhancement; clinical trial; pandemic preparedness; vaccine.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. The author reports no conflicts of interest in this work. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Thanh Le T, Andreadakis Z, Kumar A, et al. The COVID-19 vaccine development landscape. Nature Reviews Drug Discovery 2020; 19:305–6. - PubMed
-
- Yamey G, Gordon R, Gray GE. Pandemic vaccine trials in low- and middle-income countries and global health security. JAMA Network Open 2021; 4:e2134455. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

