Hybrid Immunity in a Mozambican Cohort After 1 or 2 Doses of the BBIBP-CorV Vaccine
- PMID: 40694517
- PMCID: PMC12282517
- DOI: 10.1093/cid/ciaf095
Hybrid Immunity in a Mozambican Cohort After 1 or 2 Doses of the BBIBP-CorV Vaccine
Abstract
Background: More than half of the BBIBP-CorV vaccines, outside of Pacific Asia, were distributed in Africa. Nevertheless, there are limited data on the immunogenicity of BBIBP-CorV from Africa. We compared the antibody response, after 1 and 2 doses of the BBIBP-CorV vaccine, in individuals seropositive or seronegative to severe acute respiratory syndrome coronavirus 2 prior to vaccination.
Methods: From March to May 2021, blood samples were obtained at first and second doses of the BBIBP-CorV, and 2 weeks later. Antibody titers against the full-length spike, receptor binding domain and nucleocapsid protein (anti-NC) of severe acute respiratory syndrome coronavirus 2 were measured. Pseudovirus neutralization assays and antibody-dependent cellular cytotoxicity (ADCC) against the D614G, BA.2, and BA.4 variants were also evaluated.
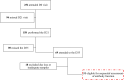
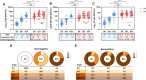
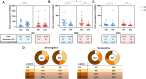
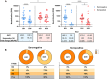
Results: At the second dose, the immunoglobulin G titers for full-length spike and anti-nucleocapsid protein, the ADCC against BA-2, and the neutralizing activity against the D614G and BA.2 were higher in individuals seropositive to any of the epitopes at the first dose (n = 26) compared to the levels observed 2 weeks later in the seronegative group (n = 25). We did not observe an increase on magnitude of binding antibodies, ADCC, and neutralizing activities, in those seropositive, after the second homologous dose of the BBIBP-CorV vaccine.
Conclusions: We suggest that 1 dose of the BBIBP-CorV vaccine in seropositive individuals induced better antibodies response including against variant of concerns compared to that observed after 2 doses in seronegative individuals. A further homologous dose of the BBIBP-CorV vaccine, in those who are seropositive, does not improve the antibody response observed after the first dose.
Keywords: BBIBP-CorV; COVID-19 vaccine; antibodies; hybrid immunity; inactivated vaccine.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Figures
References
-
- WHO . WHO—Prequalification of Medical Products (IVDs, Medicines, Vaccines and Immunization Devices, Vector Control). COVID-19 Vaccines with WHO Emergency Use Listing 2022. Available at: https://extranet.who.int/pqweb/vaccines/vaccinescovid-19-vaccine-eul-issued. Accessed 30 November 2022.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

