STAT1-mediated interferon signaling in the hematopoietic system is essential for restricting Usutu virus infection in vivo
- PMID: 40694555
- PMCID: PMC12282914
- DOI: 10.1371/journal.pntd.0013317
STAT1-mediated interferon signaling in the hematopoietic system is essential for restricting Usutu virus infection in vivo
Abstract
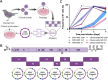
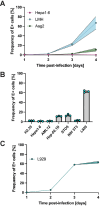
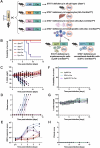
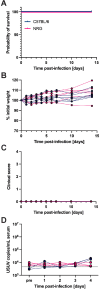
Usutu virus (USUV) is an emerging mosquito-borne flavivirus known to induce neuroinvasive disease in birds, mice, and humans in European and African countries. The mechanisms of infection and dissemination remain poorly understood. Thus, elucidating how USUV spreads in a susceptible host is crucial for identifying therapeutic targets. To investigate host defenses against USUV, we generated an infectious clone of the TC508 isolate. After characterizing its replication dynamics in cultured cells from multiple species, we investigated its pathogenesis in an array of mice with genetic perturbations. Previous studies demonstrated that whole-body deletion of type I interferon (IFN) signaling led to widespread USUV infection and fatality in mice. Here, we observed the same lethal phenotype in STAT1-deficient mice and identified hematopoietic cells specifically as central to USUV pathogenesis in a mammalian host. Deletion of STAT1 in all hematopoietic subsets, but not hepatocytes, neurons, macrophages or conventional dendritic cells, was sufficient for systemic viral dissemination and ultimate fatality. Conversely, mice lacking functional B, T, and natural killer (NK) cells but with intact myeloid cells were resistant to USUV. Our findings provide new insights into the tissue-specific barriers that regulate USUV infection and underscore the importance of innate immunity in host defense for this important emerging flavivirus.
Copyright: © 2025 Nelson et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors of this manuscript have read the journal’s policy and declare the following competing interests. R.E.S. is on the scientific advisory boards of Miromatrix Inc. and Lime Therapeutics and is a speaker and consultant for Alnylam Inc.
Figures


Similar articles
-
The segmented flavivirus Alongshan virus reduces mitochondrial mass by degrading STAT2 to suppress the innate immune response.J Virol. 2025 Jan 31;99(1):e0130124. doi: 10.1128/jvi.01301-24. Epub 2024 Dec 10. J Virol. 2025. PMID: 39655955 Free PMC article.
-
A MicroRNA Screen Identifies the Wnt Signaling Pathway as a Regulator of the Interferon Response during Flavivirus Infection.J Virol. 2017 Mar 29;91(8):e02388-16. doi: 10.1128/JVI.02388-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28148804 Free PMC article.
-
Cross-protection against St. Louis encephalitis virus and Usutu virus by West Nile virus convalescent plasma.Virology. 2025 Jul;608:110555. doi: 10.1016/j.virol.2025.110555. Epub 2025 Apr 18. Virology. 2025. PMID: 40273513
-
Extrapolation and comparison of West Nile virus- and Usutu virus-associated neurological diseases in humans: linking pathology to clinical symptoms.Clin Microbiol Rev. 2025 Jul 1:e0023224. doi: 10.1128/cmr.00232-24. Online ahead of print. Clin Microbiol Rev. 2025. PMID: 40590537 Review.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
References
-
- McIntosh BM. Usutu (SA Ar 1776), nouvel arbovirus du groupe B. Int Catalogue Arboviruses. 1985;3:1059–60.
-
- Williams MC, Simpson DI, Haddow AJ, Knight EM. The isolation of West Nile virus from man and of Usutu virus from the bird-biting mosquito Mansonia aurites (Theobald) in the Entebbe area of Uganda. Ann Trop Med Parasitol. 1964;58:367–74. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

