A chaperonin complex regulates organelle proteostasis in malaria parasites
- PMID: 40694565
- PMCID: PMC12282863
- DOI: 10.1371/journal.ppat.1013275
A chaperonin complex regulates organelle proteostasis in malaria parasites
Abstract
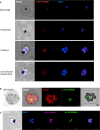
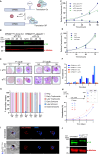
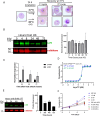
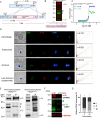
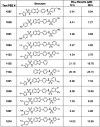
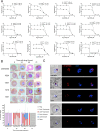
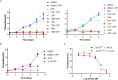
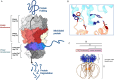
The apicoplast of Plasmodium parasites serves as a metabolic hub that synthesize essential biomolecules. Like other endosymbiotic organelles, 90% of the apicoplast proteome is encoded by the cell nucleus and transported to the organelle. Evidence suggests that the apicoplast has minimal control over the synthesis of its proteome and therefore it is unclear how organelle proteostasis is regulated. Here, we identified and investigated a large and conserved chaperonin (CPN) complex with a previously unknown function. Using genetic tools, we demonstrated that ablation of the apicoplast CPN60 subunit leads to parasite death due to organellar damage, immediately within its first replication cycle, deviating from the delayed death phenotype commonly observed for apicoplast translation inhibitors. Unlike its close orthologues in other prokaryotic and eukaryotic cells, CPN60 is not upregulated during heat shock (HS) and does not affect HS response in the parasite. Instead, we found that it is directly involved in proteostasis through interaction with the Clp (caseinolytic protease) proteolytic complex. We showed that CPN60 physically binds both the active and inactive forms of the Clp complex, and manipulates its stability. A computational structural model of a possible interaction between these two large complexes suggests a stable interface. Finally, we screened a panel of inhibitors for the bacterial CPN60 orthologue GroEL, to test the potential of chaperonin inhibition as antimalarial. These inhibitors demonstrated an anti-Plasmodium activity that was not restricted to apicoplast function, with additional targets outside of this organelle. Taken together, this work reveals how balanced activities of proteolysis and refolding safeguard the apicoplast proteome, and are essential for organelle biogenesis.
Copyright: © 2025 Tissawak et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and an author of this manuscript have the following competing interests: S.M.J. is a founder of BioEL Inc, which was formed to commercialize GroEL inhibitors for antibacterial applications.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

