Busulfan damages spermatogenic function by inducing orchitis
- PMID: 40694571
- PMCID: PMC12282903
- DOI: 10.1371/journal.pone.0322721
Busulfan damages spermatogenic function by inducing orchitis
Abstract
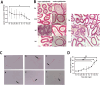
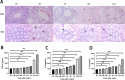
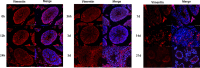
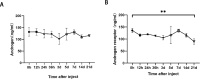
In order to clarify injure mechanism of busulfan to spermatogenic function, we treated mice with busulfan, the testicular and epididymal weights and sperm concentration significantly decreased and the sperm malformation rate increased over time. Moreover, testicular interstitial cell infiltration, a smaller seminiferous tubule, and disorganized and shed spermatogenic cells were also observed by immunohistochemical, immunofluorescence detection after the busulfan treatment. Furthermore, the enzyme-linked absorbance assays showed serum interleukin (IL)-6, IL-1β, and tumor necrosis factor-apha levels (inflammatory factors) were significantly upregulated; blood-testis barrier (BTB)-related protein levels (e.g., N-Cadherin, occludin, and connexin 43) and vimentine gradually decreased. So we infer busulfan treatment induced orchitis, further disrupted the BTB and disrupted the spermatogenic microenvironment, then decreased vimentine and gradually damaged the cytoskeleton, which cause spermatogenic cells losing their supporting from sertoli cells, androgen regulation was also affected, which was detrimental to spermatogenesis. The study result will improve the efficiency and safety in spermatogonial stem cell transplant recipients.
Copyright: © 2025 Zhao et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Choderlos de Laclos X, Risbourg S, Brennan B, Bertucci F, Gaspar N, Gelderblom H, et al. Impact of age on safety of Busulfan-Melphalan followed by autologous hematopoietic stem-cell transplantation versus standard chemotherapy in the patients of the EURO-E.W.I.N.G. 99 and Ewing 2008 clinical trials. Eur J Cancer. 2024;208:114229. doi: 10.1016/j.ejca.2024.114229 - DOI - PMC - PubMed
-
- Duléry R, Bastos J, Paviglianiti A, Malard F, Brissot E, Battipaglia G, et al. Thiotepa, Busulfan, and Fludarabine Conditioning Regimen in T Cell-Replete HLA-Haploidentical Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2019;25(7):1407–15. doi: 10.1016/j.bbmt.2019.02.025 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

