Ceragenin Nanogel Coating Prevents Biofilms Formation on Urinary Catheters
- PMID: 40694658
- PMCID: PMC12332827
- DOI: 10.1021/acsami.5c13979
Ceragenin Nanogel Coating Prevents Biofilms Formation on Urinary Catheters
Abstract
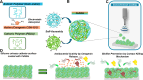
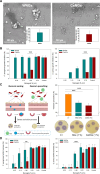
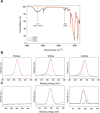
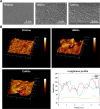
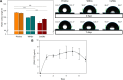
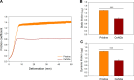
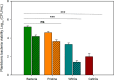
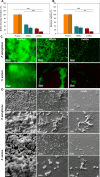
Catheter-associated urinary tract infections (CAUTIs) account for 40% of hospital-acquired infections, increasing health risks, patient discomfort, morbidity, and hospitalisation time. Bacterial colonisation may occur both during catheter insertion and prolonged catheterization by microorganisms in the urinary tract, consequently, increasing the bacteriuria risk due to biofilm formation. Nanogels, a class of soft nanocarriers, offer remarkable versatility for developing functional coatings on indwelling medical devices that can efficiently prevent biofilm formation. In this study, we designed an antibacterial and antibiofilm coating by leveraging the ultrasound-assisted assembly and nanogel self-organization on silicone catheters. The nanogel comprising biocompatible gum arabic and poly(diallyldimethylammonium chloride) was used to encapsulate a synthetic broad-spectrum antimicrobial peptide mimetic ceragenin (CSA-131). A coating from this bioactive nanogel was sonochemically built on the catheters without any prior surface modification. In vitro and in vivo assays showed that the coating provided antimicrobial and antibiofilm activity for up to 7 days of catheterization, next to catheter lubricity. Cytotoxicity assessment confirmed the absence of toxic effects, underscoring the biocompatibility of the coating formulation. These findings highlighted the potential of nanogels, combined with ultrasound technology, as an innovative approach for durable antimicrobial and antibiofilm functionalization of urinary catheters, particularly susceptible to colonisation by microorganisms upon catheterization.
Keywords: antimicrobial and antibiofilm coating; ceragenin; nanogels; self-assembly; ultrasound; urinary catheters.
Figures


Similar articles
-
Types of indwelling urethral catheters for short-term catheterisation in hospitalised adults.Cochrane Database Syst Rev. 2014 Sep 23;2014(9):CD004013. doi: 10.1002/14651858.CD004013.pub4. Cochrane Database Syst Rev. 2014. PMID: 25248140 Free PMC article.
-
LubriShieldTM-A permanent urinary catheter coating that prevents uropathogen biofilm formation in vitro independent of host protein conditioning.PLoS One. 2025 Jul 10;20(7):e0328167. doi: 10.1371/journal.pone.0328167. eCollection 2025. PLoS One. 2025. PMID: 40638652 Free PMC article.
-
Types of urethral catheters for management of short-term voiding problems in hospitalised adults.Cochrane Database Syst Rev. 2004;(1):CD004013. doi: 10.1002/14651858.CD004013.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2008 Apr 16;(2):CD004013. doi: 10.1002/14651858.CD004013.pub3. PMID: 14974052 Updated.
-
Long-Term Prevention of Biofilm Formation by Polycatechol-Based Supramolecular Assemblies with Low Molecular Weight Polymers on Surfaces.ACS Appl Mater Interfaces. 2024 Jul 24;16(29):38631-38644. doi: 10.1021/acsami.4c02371. Epub 2024 Jul 9. ACS Appl Mater Interfaces. 2024. PMID: 38980701 Free PMC article.
-
3D-printed porous titanium rods equipped with vancomycin-loaded hydrogels and polycaprolactone membranes for intelligent antibacterial drug release.Sci Rep. 2024 Sep 18;14(1):21749. doi: 10.1038/s41598-024-72457-1. Sci Rep. 2024. PMID: 39294268 Free PMC article.
Cited by
-
Bottom-up sono-enzymatic approach to build antimicrobial and antifouling nano-enabled coatings on urinary catheters.Nanoscale Adv. 2025 Aug 5. doi: 10.1039/d5na00577a. Online ahead of print. Nanoscale Adv. 2025. PMID: 40801046 Free PMC article.
References
-
- Trizna E. Y., Yarullina M. N., Baidamshina D. R., Mironova A. V., Akhatova F. S., Rozhina E. V., Fakhrullin R. F., Khabibrakhmanova A. M., Kurbangalieva A. R., Bogachev M. I., Kayumov A. R.. Bidirectional Alterations in Antibiotics Susceptibility in Staphylococcus Aureus–Pseudomonas Aeruginosa Dual-Species Biofilm. Sci. Rep. 2020;10(1):14849. doi: 10.1038/s41598-020-71834-w. - DOI - PMC - PubMed
-
- Andersen M. J., Flores-Mireles A. L.. Urinary Catheter Coating Modifications: The Race against Catheter-Associated Infections. Coatings. 2019;10(1):23–25. doi: 10.3390/coatings10010023. - DOI
-
- Duan Q. Y., Zhu Y. X., Jia H. R., Wang S. H., Wu F. G.. Nanogels: Synthesis, Properties, and Recent Biomedical Applications. Prog. Mater. Sci. 2023;139(July):101167. doi: 10.1016/j.pmatsci.2023.101167. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

