KFLC to Total CSF Protein Ratio Is an Alternative to KFLC Index to Diagnose Multiple Sclerosis: A Retrospective Study of 814 Cases
- PMID: 40694730
- PMCID: PMC12285672
- DOI: 10.1212/NXI.0000000000200451
KFLC to Total CSF Protein Ratio Is an Alternative to KFLC Index to Diagnose Multiple Sclerosis: A Retrospective Study of 814 Cases
Abstract
Background and objectives: Biological analyses are crucial for diagnosing multiple sclerosis (MS), notably detection of oligoclonal bands (OCBs) of immunoglobulin G in CSF. Kappa free light chain (KFLC) quantification in serum and CSF has recently been proposed as an additional test. In a rational medicoeconomic approach, identifying algorithms or biomarkers is important. The objective of this study was, therefore, to evaluate the correlation between CSF kappa free light chain (KFLCCSF)/total CSF protein ratio (KFLCCSF/ProtCSF) and OCB status and its performance in diagnosing MS.
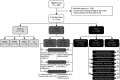
Methods: KFLC measurements were performed prospectively with each OCB analysis, and the data were interpreted retrospectively. A total of 814 clinical cases were included: 153 with MS or clinically isolated syndrome (CIS), 181 with other inflammatory neurologic diseases, and 480 with noninflammatory neurologic diseases. Performances of KFLCCSF/ProtCSF in predicting OCB status, diagnosing MS, and estimating evolution of CIS to MS were evaluated and compared with those of other KFLC parameters, i.e., KFLC index, and with OCB and IgG parameters.
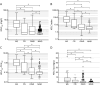
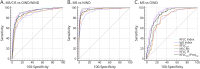
Results: KFLCCSF/ProtCSF and KFLC index performed similarly in predicting OCB status, with a sensitivity of 80.2% and a specificity of 93.4% at a threshold >0.21% for KFLCCSF/ProtCSF (area under the curve: 0.93). The percentage of agreement between OCB status and KFLCCSF/ProtCSF was 91.2%. Using KFLCCSF and KFLCCSF/ProtCSF initially could reduce OCB testing by 68% and quantitative tests by 48.4%. For diagnosing MS/CIS, KFLCCSF/ProtCSF performed similar to or slightly worse than the KFLC index but always outperformed IgG, OCB, and KFLCCSF. For this indication, the optimal threshold for KFLCCSF/ProtCSF was >0.24%. It is important to note that KFLCCSF/ProtCSF was the most predictive parameter for CIS progression to MS.
Discussion: To conclude, KFLCCSF/ProtCSF is as an easier and cost-effective alternative for predicting OCB status and CIS progression to MS and assisting in the diagnosis of MS.
Classification of evidence: This study provides Class II evidence that the KFLCCSF/ProteinCSF ratio accurately distinguishes patients with CSF OCBs from those without CSF OCBs and patients with MS from those with other neurologic disorders.
Conflict of interest statement
E. Moschetti, M. Venet, L. Thibaudin, and A. Moreau report no disclosures; P. Gonzalo declare no competing interests. Y. Tholance reports personal fees from The Binding Site and Alexion, outside of the submitted work. J.P. Camdessanché reports personal fees for lectures, consulting, writing of articles, or training courses from Akcea, Alnylam, Biogen, CSL Behring, Genzyme, Laboratoire Français des Biotechnologies, Merck, Novartis, Pfizer, Pharmalliance, Teva, Editions Scientifiques L&C, Edimark, Expression Santé, Natus, Scien, SNF-Floerger, outside of the submitted work, and is holding a patent on anti-fibroblast growth factor receptor 3 (FGFR3) and argonaute (AGO) autoantibodies. Go to
Figures


References
-
- Association des Collèges des Enseignants d'Immunologie des Universités de Langue française. Item 125: Sclérose en plaques 2011 2012.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
