Seeing Is Believing: How Does the Surface of Silver Nanocubes from a Polyol Synthesis Change during Sample Collection, Washing, and Redispersion
- PMID: 40694844
- PMCID: PMC12333423
- DOI: 10.1021/acs.langmuir.5c02610
Seeing Is Believing: How Does the Surface of Silver Nanocubes from a Polyol Synthesis Change during Sample Collection, Washing, and Redispersion
Abstract
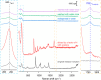
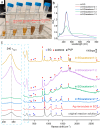
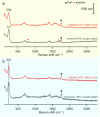
While the synthesis of Ag nanocubes has been extensively studied, sample preparation (including collection, washing, and redispersion) after the synthesis has received far less attention. Herein, we leverage the unique capability of surface-enhanced Raman scattering to investigate how the solvent used for sample preparation affects the surface chemistry of Ag nanocubes. Our findings reveal that the use of an appropriate solvent for sample preparation plays a vital role in preserving the cubic shape. Crushing the reaction mixture with acetone before centrifugation greatly improves collection efficiency by inducing reversible aggregation among the particles. It also promotes the coadsorption of the carbonyl group from acetone and Cl- ions on the Ag surface to suppress oxidative etching and thereby help preserve the cubic shape. Subsequent washing of the collected nanocubes with water or ethanol enables effective redispersion while facilitating the desorption of Cl- ions and the adsorption of the carbonyl group from poly(vinylpyrrolidone). Collectively, these results underscore the importance of processing conditions after a colloidal synthesis in preserving the desired properties of Ag nanocubes for an array of applications.
Figures


References
LinkOut - more resources
Full Text Sources

