The extended mobility of plasmids
- PMID: 40694848
- PMCID: PMC12282955
- DOI: 10.1093/nar/gkaf652
The extended mobility of plasmids
Abstract
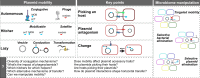
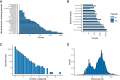
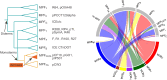
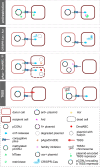
Plasmids play key roles in the spreading of many traits, ranging from antibiotic resistance to varied secondary metabolism, from virulence to mutualistic interactions, and from defense to antidefense. Our understanding of plasmid mobility has progressed extensively in the last few decades. Conjugative plasmids are still often the textbook image of plasmids, yet they are now known to represent a minority. Many plasmids are mobilized by other mobile genetic elements, some are mobilized as phages, and others use atypical mechanisms of transfer. This review focuses on recent advances in our understanding of plasmid mobility, from the molecular mechanisms allowing transfer and evolutionary changes of plasmids to the ecological determinants of their spread. In this emerging, extended view of plasmid mobility, interactions between mobile genetic elements, whether involving exploitation, competition, or elimination, affect plasmid transfer and stability. Likewise, interactions between multiple cells and their plasmids shape the latter patterns of transfer through transfer-mediated bacterial predation, interference, or eavesdropping in cell communication, and by deploying defense and antidefense activity. All these processes are relevant for microbiome intervention strategies, from plasmid containment in clinical settings to harnessing plasmids in ecological or industrial interventions.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Conflict of interest statement
None declared.
Figures



References
-
- Arnold BJ, Huang I, Hanage WP Horizontal gene transfer and adaptive evolution in bacteria. Nat Rev Micro. 2021; 30:206–18. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

