Identification of regulatory sequences in Aca11 and Aca13 for detection of anti-CRISPR and protein-protein interaction
- PMID: 40694851
- PMCID: PMC12282945
- DOI: 10.1093/nar/gkaf694
Identification of regulatory sequences in Aca11 and Aca13 for detection of anti-CRISPR and protein-protein interaction
Abstract
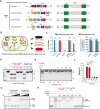
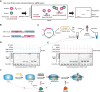
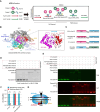
Anti-CRISPR (Acr) proteins are frequently co-encoded with the anti-CRISPR associated (Aca) proteins, which act as repressors for regulating Acr expression within acr-aca operons. We previously identified three aca genes (aca11-13) from Streptococcus mobile genetic elements, but their regulatory mechanisms remained unclear. Here, we showed that Aca11 and Aca13 mediate bidirectional regulation in acr-aca operons through recognition of their inverted repeat (IR) sequences within the acr promoters. Based on the bioinformatics search using Aca13 with its IR sequences, we discovered a novel type II-A Acr (named AcrIIA35). AcrIIA35 exhibits a potent inhibitory activity against St1Cas9 by interfering with DNA recognition of Cas9 in bacterial and human cells. We also developed a novel Aca-driven protein-protein interaction detection (APID) system by integrating Aca-tagged target proteins with fluorescently labeled IR-DNA probes. The APID system enables efficient detection of protein-protein interaction using proteins or crude cell lysates. Utilizing the APID system, we have further elucidated the mechanism of AcrIIA24, which can interact with the HNH nuclease domain of St3Cas9 to inhibit the DNA cleavage activity of Cas9. Collectively, our work expands the understanding of Aca functions to modulate Acrs and expands the potential for Aca-based applications in CRISPR technologies.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Conflict of interest statement
None declared.
Figures




Similar articles
-
Insights on the regulation and function of the CRISPR/Cas transposition system located in the pathogenicity island VpaI-7 from Vibrio parahaemolyticus RIMD2210633.Infect Immun. 2025 Jun 10;93(6):e0016925. doi: 10.1128/iai.00169-25. Epub 2025 May 1. Infect Immun. 2025. PMID: 40310292 Free PMC article.
-
Application of CHyMErA Cas9-Cas12a combinatorial genome-editing platform for genetic interaction mapping and gene fragment deletion screening.Nat Protoc. 2021 Oct;16(10):4722-4765. doi: 10.1038/s41596-021-00595-1. Epub 2021 Sep 10. Nat Protoc. 2021. PMID: 34508260 Free PMC article. Review.
-
Evolutionary trends in Bombella apis CRISPR-Cas systems.mSystems. 2025 Jul 22;10(7):e0016625. doi: 10.1128/msystems.00166-25. Epub 2025 Jun 18. mSystems. 2025. PMID: 40530883 Free PMC article.
-
Type I-E* CRISPR-Cas of Klebsiella pneumoniae upregulates bacterial virulence by targeting endogenous histidine utilization system.mSphere. 2025 Jun 25;10(6):e0021525. doi: 10.1128/msphere.00215-25. Epub 2025 May 19. mSphere. 2025. PMID: 40387367 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

