Reduced circulating NrCAM as a biomarker for fetal growth restriction
- PMID: 40694862
- PMCID: PMC12301788
- DOI: 10.1016/j.ebiom.2025.105854
Reduced circulating NrCAM as a biomarker for fetal growth restriction
Abstract
Background: Placental insufficiency underpins pregnancy complications, fetal growth restriction (FGR) and preeclampsia, yet predictive biomarkers are limited. Neuronal Cell Adhesion Molecule (NrCAM) may be a promising biomarker of placental dysfunction. This study investigated whether NrCAM can predict diseases of placental insufficiency.
Methods: Circulating NrCAM was measured across independent cohorts. Plasma NrCAM was assessed at 36 weeks' gestation in women who later delivered FGR infants (<3rd centile birthweight), or developed preeclampsia at term. Circulating NrCAM was also measured in international cohorts: a UK high-risk cohort of women presenting with reduced fetal movements and delivered an FGR infant; a high-risk cohort from South Africa diagnosed with preeclampsia or eclampsia. NrCAM was also assessed in pregnancies with preterm FGR or preeclampsia (<34 weeks gestation). The effect of hypoxia on NrCAM expression was measured in trophoblast stem cells, primary trophoblasts, and a murine FGR model.
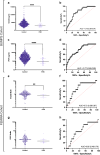
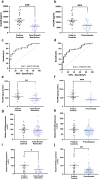
Findings: Circulating NrCAM was reduced at 36 weeks' gestation in women who later delivered FGR infants (p = 4.75 x 10-6, AUC = 0.76, n = 26 FGR, n = 957 controls). In the UK cohort, reduced NrCAM levels were associated with FGR (p = 9.34 × 10-3, AUC = 0.72, n = 12 FGR, n = 235 control). In the South Africa cohort, circulating NrCAM was reduced with preeclampsia (p = 0.03, AUC = 0.70, n = 27 preeclampsia, n = 15 control). Placental NrCAM expression was lower in FGR (p = 0.0003, n = 23 FGR) and preeclampsia (p = 0.0003, n = 41 preeclampsia, n = 20 controls). Hypoxia reduced NrCAM expression in human trophoblast stem cells (p < 0.01) primary trophoblasts (p < 0.0001) and in a murine FGR model (p < 0.01, n = 9 per group).
Interpretation: Reductions in plasma and placental NrCAM are strongly associated with FGR and may be driven by hypoxia.
Funding: This study was funded by a grant from National Health and Medical Research Council.
Keywords: Biomarker; FGR; Hypoxia; NrCAM; Placenta; Preeclampsia.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests LB: received research grants from the Swedish Research Council, STINT, Märta Lundqvist stiftelse, Swedish Society of Medicine, Gothenburg Society of Medicine, SSMF, Jane and Dan Olssons stiftelse, Swedish Brain fund, Jeanssons stiftelse, Wallenberg Centre for Molecular and Translational Medicine and the European Research Council. LB has also obtained reimbursement for lecture by iLab Medical and reimbursement as expert opionion from Homburg and Partner; Thermo Fisher Preeclampsia symposium, Uppsala, 2024; invited speaker at SRI, Global Obstetric Update, EAPM, NFOG, ISSHP. LB is also a board member responsible for the biobank in the IMPACT study where PlGF reagents have been donated by Roche, PerkinElmer and Thermo Fischer. Course leader for the course in Preeclamspia in Sweden with sponsorship by Thermo Fischer and Roche. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Interventions affecting the nitric oxide pathway versus placebo or no therapy for fetal growth restriction in pregnancy.Cochrane Database Syst Rev. 2023 Jul 10;7(7):CD014498. doi: 10.1002/14651858.CD014498. Cochrane Database Syst Rev. 2023. PMID: 37428872 Free PMC article.
-
Use of biochemical tests of placental function for improving pregnancy outcome.Cochrane Database Syst Rev. 2015 Nov 25;2015(11):CD011202. doi: 10.1002/14651858.CD011202.pub2. Cochrane Database Syst Rev. 2015. PMID: 26602956 Free PMC article.
-
The association between circulating SIGLEC6 and preeclampsia: observational studies of seven cohorts.EBioMedicine. 2025 Aug;118:105870. doi: 10.1016/j.ebiom.2025.105870. Epub 2025 Jul 29. EBioMedicine. 2025. PMID: 40737757 Free PMC article.
-
The 36-week preeclampsia risk by the Fetal Medicine Foundation algorithm is associated with fetal compromise following induction of labor.Am J Obstet Gynecol. 2025 Jul;233(1):57.e1-57.e12. doi: 10.1016/j.ajog.2024.12.025. Epub 2024 Dec 24. Am J Obstet Gynecol. 2025. PMID: 39725374
-
Planned early delivery versus expectant management of the term suspected compromised baby for improving outcomes.Cochrane Database Syst Rev. 2015 Nov 24;2015(11):CD009433. doi: 10.1002/14651858.CD009433.pub2. Cochrane Database Syst Rev. 2015. PMID: 26599471 Free PMC article.
References
-
- Audette M.C., Kingdom J.C. Screening for fetal growth restriction and placental insufficiency. Semin Fetal Neonatal Med. 2018;23(2):119–125. WB Saunders. - PubMed
-
- Lawn J.E., Blencowe H., Waiswa P., et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. 2016;387(10018):587–603. - PubMed
-
- Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–137. WB Saunders. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

