High-fat diet obesity exacerbates acute lung injury-induced dysregulation of fatty acid oxidation in alveolar epithelial type 2 cells
- PMID: 40695588
- PMCID: PMC12352525
- DOI: 10.1152/ajplung.00406.2024
High-fat diet obesity exacerbates acute lung injury-induced dysregulation of fatty acid oxidation in alveolar epithelial type 2 cells
Abstract
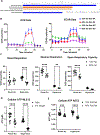
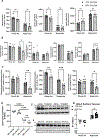
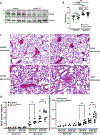
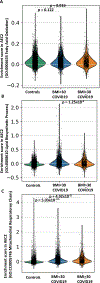
Obesity is a risk factor for acute respiratory distress syndrome (ARDS). We previously showed that obesity is linked to increased lung injury and bronchoalveolar lavage fluid (BALF) fatty acids in a hyperoxic model of ARDS. We sought to expand our understanding of this association and examined the effect of obesity on β-oxidation (FAO), the mitochondrial process of breaking down fatty acids, in alveolar epithelial type 2 cells (AEC2s) in hyperoxia-induced ARDS. AEC2 were isolated from mice receiving 60% versus 10% fat diet. Carnitine palmitoyltransferase 1A (CPT1A) mediates the transport of fatty acids into mitochondria for subsequent FAO. Cpt1aloxp/loxpSftpcCreERT2+/- mice were generated with AEC2-specific CPT1A downregulation. Obesity was associated with intracellular lipid accumulation and increased expression of CPT1A in AEC2 after hyperoxia. Mitochondrial FAO; however, was significantly transcriptionally downregulated in AEC2 of obese mice compared with lean mice after hyperoxia. AEC2 from obese mice exhibited more severe mitochondrial bioenergetic failure and reduced ATP production after hyperoxia compared with lean mice. Consistent with earlier reports linking FAO perturbation to surfactant impairment, we also observed that high-fat diet was associated with reduced surfactant-related phospholipids in hyperoxic AEC2 and increased BALF surface tension, although obese Cpt1aloxp/loxpSftpcCreERT2+/- mice were not protected from increased lung injury. In a reanalysis of a human single-cell lung atlas of COVID-19 ARDS, the downregulation of the FAO signature in AEC2 was significant only in obese, and not lean, patients with ARDS compared with controls. These findings demonstrate a previously underappreciated effect of diet on AEC2 function in acute lung injury.NEW & NOTEWORTHY High-fat diet obesity is linked to increased lung injury and bronchoalveolar lavage fluid (BALF) fatty acids in a hyperoxic ARDS model. In the present study, obesity not only upregulated intracellular lipids and effectors of fatty acid mitochondrial import but also was associated with downregulated fatty acid oxidation and reduced ATP production in alveolar epithelial type 2 cells after injury. Hyperoxic AEC2 from obese mice had reduced phospholipids, and obese mice had increased BALF surface tension after injury.
Keywords: ARDS; acute lung injury; fatty acid oxidation; lipid metabolism; obesity.
Conflict of interest statement
Figures

Comment in
-
Are alveolar type 2 cells "oversaturated" in obesity and ARDS?Am J Physiol Lung Cell Mol Physiol. 2025 Nov 1;329(5):L612-L613. doi: 10.1152/ajplung.00325.2025. Epub 2025 Oct 14. Am J Physiol Lung Cell Mol Physiol. 2025. PMID: 41087035 No abstract available.
References
-
- Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, and Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA 307: 2526–2533, 2012. - PubMed
-
- Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, Brenner SK, Leonberg-Yoo A, Schenck EJ, Radbel J, Reiser J, Bansal A, Srivastava A, Zhou Y, Sutherland A, Green A, Shehata AM, Goyal N, Vijayan A, Velez JCQ, Shaefi S, Parikh CR, Arunthamakun J, Athavale AM, Friedman AN, Short SAP, Kibbelaar ZA, Abu Omar S, Admon AJ, Donnelly JP, Gershengorn HB, Hernan MA, Semler MW, Leaf DE, and Investigators S-C. Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US. JAMA Intern Med 180: 1436–1447, 2020. - PMC - PubMed
-
- Lewandowski K, and Lewandowski M. Intensive care in the obese. Best Pract Res Clin Anaesthesiol 25: 95–108, 2011. - PubMed
-
- Fezeu L, Julia C, Henegar A, Bitu J, Hu FB, Grobbee DE, Kengne AP, Hercberg S, and Czernichow S. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta-analysis. Obes Rev 12: 653–659, 2011. - PubMed
MeSH terms
Substances
Grants and funding
- K08HL157728/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- 5U24DK137318/HHS | NIH | NIDDK | Division of Diabetes, Endocrinology, and Metabolic Diseases (DEM)
- 2T32HL134629-06A1/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- U24 DK137318/DK/NIDDK NIH HHS/United States
- K HL-165081/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- P30 CA008748/CA/NCI NIH HHS/United States
- K23 GM151730/GM/NIGMS NIH HHS/United States
- K23 GM151730-01/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- K08 HL157728/HL/NHLBI NIH HHS/United States
- K08 HL165081/HL/NHLBI NIH HHS/United States
- T32 HL134629/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

